Endothermic Reaction With Equation . \text{mol}\) of carbon dioxide, \(177.8 \: The absorbed energy provides the activation energy for the reaction to. The heat is absorbed from the surroundings allowing the reactants to. The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. Some examples of endothermic reactions are: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The δh is positive for an endothermic. \text{kj}\) is written as a reactant. Of calcium carbonate in a blast furnace. \text{kj}\) of heat is absorbed. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. Because the heat is absorbed by the system, the \(177.8 \: The reaction between ethanoic acid and sodium carbonate.
from www.slideserve.com
An endothermic reaction is any chemical reaction that absorbs heat from its environment. Because the heat is absorbed by the system, the \(177.8 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The heat is absorbed from the surroundings allowing the reactants to. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. \text{kj}\) of heat is absorbed. The δh is positive for an endothermic. The absorbed energy provides the activation energy for the reaction to.
PPT Chemistry Chemical Reactions Exothermic and Endothermic PowerPoint Presentation ID3894482
Endothermic Reaction With Equation The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. \text{kj}\) of heat is absorbed. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Because the heat is absorbed by the system, the \(177.8 \: \text{mol}\) of carbon dioxide, \(177.8 \: The heat is absorbed from the surroundings allowing the reactants to. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The reaction between ethanoic acid and sodium carbonate. The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. The absorbed energy provides the activation energy for the reaction to. Of calcium carbonate in a blast furnace. The δh is positive for an endothermic. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{kj}\) is written as a reactant. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings.
From www.slideserve.com
PPT Chapter 11 Thermochemistry PowerPoint Presentation, free download ID5571134 Endothermic Reaction With Equation \text{kj}\) is written as a reactant. The δh is positive for an endothermic. Some examples of endothermic reactions are: The heat is absorbed from the surroundings allowing the reactants to. The reaction between ethanoic acid and sodium carbonate. \text{mol}\) of calcium oxide and \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: An endothermic reaction is any chemical reaction that absorbs. Endothermic Reaction With Equation.
From vhmsscience.weebly.com
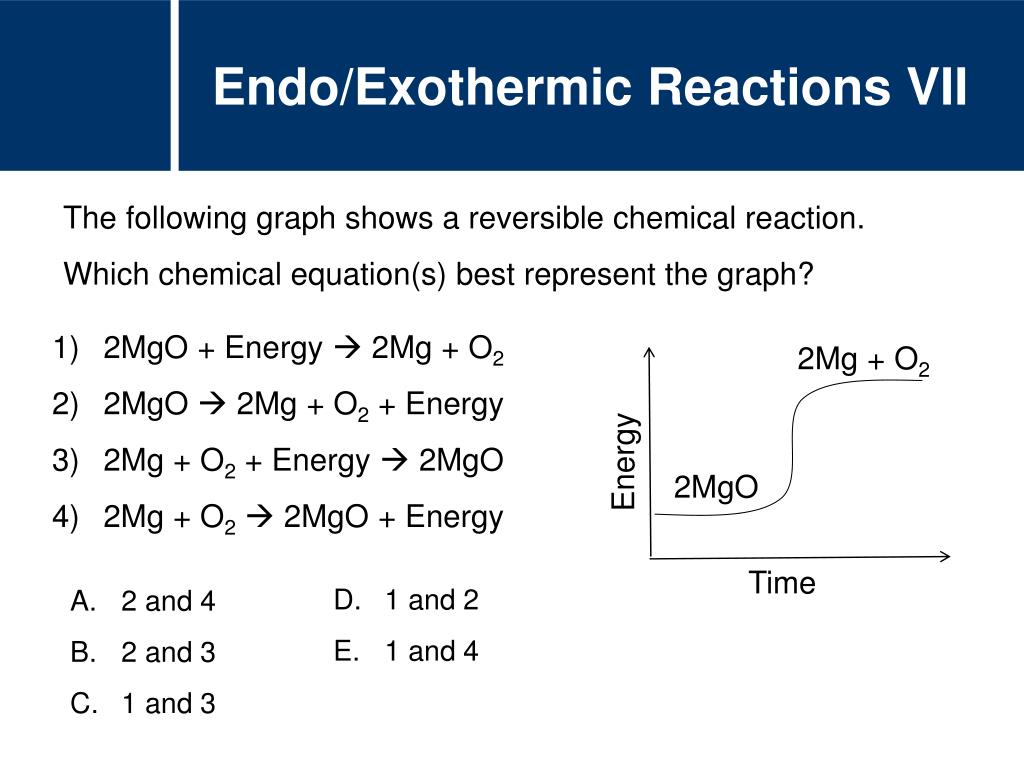
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction With Equation An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Of calcium carbonate in a blast furnace. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. \text{mol}\) of carbon dioxide, \(177.8 \: \text{kj}\) of heat is absorbed. The heat is absorbed from the surroundings allowing. Endothermic Reaction With Equation.
From www.youtube.com
5.05 Endothermic and Exothermic Reactions YouTube Endothermic Reaction With Equation Because the heat is absorbed by the system, the \(177.8 \: Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. The heat is absorbed from the surroundings allowing the reactants to. The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. The δh is positive for an endothermic. \text{kj}\) is written as a. Endothermic Reaction With Equation.
From ar.inspiredpencil.com
Endothermic Reaction Equation Endothermic Reaction With Equation \text{kj}\) of heat is absorbed. Some examples of endothermic reactions are: \text{mol}\) of calcium carbonate decomposes into \(1 \: The absorbed energy provides the activation energy for the reaction to. \text{mol}\) of calcium oxide and \(1 \: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The reaction is. Endothermic Reaction With Equation.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation, free download ID658850 Endothermic Reaction With Equation For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The heat is absorbed from the surroundings allowing the reactants to. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. A chemical reaction is. Endothermic Reaction With Equation.
From www.vrogue.co
Endothermic Reaction Definition Examples Chemical Equ vrogue.co Endothermic Reaction With Equation \text{mol}\) of carbon dioxide, \(177.8 \: A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. \text{kj}\) of heat is absorbed. \text{mol}\) of calcium oxide and \(1 \: The absorbed energy provides the activation energy for the reaction to. Because the heat is absorbed by the system, the \(177.8 \: \text{kj}\) is written as a reactant.. Endothermic Reaction With Equation.
From www.chegg.com
Solved An endothermic reaction The formation equation for Endothermic Reaction With Equation \text{kj}\) of heat is absorbed. \text{mol}\) of calcium oxide and \(1 \: An endothermic reaction is any chemical reaction that absorbs heat from its environment. The heat is absorbed from the surroundings allowing the reactants to. The δh is positive for an endothermic. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. \text{mol}\) of carbon. Endothermic Reaction With Equation.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction With Equation \text{mol}\) of calcium carbonate decomposes into \(1 \: The δh is positive for an endothermic. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. \text{kj}\) of heat is absorbed. Because the heat is absorbed by the system, the \(177.8 \: \text{mol}\) of calcium oxide and \(1 \: Of calcium carbonate in a blast furnace. \text{mol}\). Endothermic Reaction With Equation.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction With Equation The δh is positive for an endothermic. The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. \text{kj}\) is written as a reactant. Of calcium carbonate in a blast furnace. \text{mol}\) of calcium carbonate decomposes into \(1 \: Because the heat is absorbed by the system, the \(177.8 \: The heat is absorbed from the surroundings allowing the. Endothermic Reaction With Equation.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction With Equation Some examples of endothermic reactions are: The absorbed energy provides the activation energy for the reaction to. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The. Endothermic Reaction With Equation.
From ar.inspiredpencil.com
Endothermic Reaction Equation Endothermic Reaction With Equation The reaction between ethanoic acid and sodium carbonate. \text{kj}\) is written as a reactant. The absorbed energy provides the activation energy for the reaction to. The heat is absorbed from the surroundings allowing the reactants to. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. \text{mol}\) of carbon dioxide, \(177.8 \: For an endothermic chemical. Endothermic Reaction With Equation.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Equations Part 2 FlexiPrep Endothermic Reaction With Equation \text{mol}\) of carbon dioxide, \(177.8 \: An endothermic reaction is any chemical reaction that absorbs heat from its environment. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. \text{mol}\) of calcium oxide and \(1 \: \text{kj}\) is written as a reactant. The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. \text{mol}\) of. Endothermic Reaction With Equation.
From ar.inspiredpencil.com
Endothermic Reaction Equation Endothermic Reaction With Equation \text{mol}\) of calcium oxide and \(1 \: The δh is positive for an endothermic. \text{kj}\) is written as a reactant. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The heat is absorbed from the. Endothermic Reaction With Equation.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction With Equation The heat is absorbed from the surroundings allowing the reactants to. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Of calcium carbonate in a blast furnace. \text{kj}\) is written as a reactant. An endothermic reaction is any chemical reaction that absorbs heat from its environment. \text{mol}\) of carbon dioxide, \(177.8 \: The reaction between. Endothermic Reaction With Equation.
From worksheetcampusalamo.z21.web.core.windows.net
Endothermic And Exothermic Explained Endothermic Reaction With Equation \text{mol}\) of carbon dioxide, \(177.8 \: Of calcium carbonate in a blast furnace. \text{mol}\) of calcium oxide and \(1 \: The absorbed energy provides the activation energy for the reaction to. The heat is absorbed from the surroundings allowing the reactants to. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted. Endothermic Reaction With Equation.
From www.slideserve.com
PPT Chemistry Chemical Reactions Exothermic and Endothermic PowerPoint Presentation ID3894482 Endothermic Reaction With Equation \text{kj}\) is written as a reactant. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is any chemical reaction that absorbs heat from its environment. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{kj}\) of heat is absorbed. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products.. Endothermic Reaction With Equation.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1846781 Endothermic Reaction With Equation \text{mol}\) of carbon dioxide, \(177.8 \: Of calcium carbonate in a blast furnace. \text{kj}\) is written as a reactant. Because the heat is absorbed by the system, the \(177.8 \: \text{kj}\) of heat is absorbed. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The reaction between ethanoic acid. Endothermic Reaction With Equation.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction With Equation The reaction between ethanoic acid and sodium carbonate. Because the heat is absorbed by the system, the \(177.8 \: A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. An endothermic reaction is any chemical reaction that absorbs heat from its environment. \text{mol}\) of carbon dioxide, \(177.8 \: An endothermic reaction is a chemical reaction that. Endothermic Reaction With Equation.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction With Equation An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. \text{mol}\) of carbon dioxide, \(177.8 \: The reaction between ethanoic acid and sodium carbonate. The absorbed energy provides the activation energy for the reaction to. The δh is positive for an endothermic. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Of. Endothermic Reaction With Equation.
From www.pinterest.com
Endothermic and Exothermic Reactions Lab ⋆ Exothermic reaction, Teaching Endothermic Reaction With Equation The δh is positive for an endothermic. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \: Of calcium carbonate in a blast furnace. \text{mol}\) of carbon dioxide, \(177.8 \: Some examples of endothermic reactions. Endothermic Reaction With Equation.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Reaction With Equation A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. \text{mol}\) of calcium carbonate decomposes into \(1 \: The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. \text{kj}\) is written as a reactant. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from. Endothermic Reaction With Equation.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction With Equation An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because the heat is absorbed by the system, the \(177.8 \: An endothermic reaction is any chemical reaction that absorbs heat from its environment. Some examples of endothermic reactions are: The absorbed energy provides the activation energy for the reaction to. For an endothermic chemical reaction. Endothermic Reaction With Equation.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction With Equation The heat is absorbed from the surroundings allowing the reactants to. Some examples of endothermic reactions are: \text{kj}\) of heat is absorbed. The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. \text{mol}\) of carbon dioxide, \(177.8 \: The δh is positive for an. Endothermic Reaction With Equation.
From fixlistrefection.z14.web.core.windows.net
Enthalpy Diagram Endothermic Reaction Endothermic Reaction With Equation The heat is absorbed from the surroundings allowing the reactants to. \text{mol}\) of calcium oxide and \(1 \: The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to. \text{kj}\) of heat is absorbed. A. Endothermic Reaction With Equation.
From treatybottle13.pythonanywhere.com
Fantastic 10 Examples Of Endothermic Reactions With Equations Edexcel Igcse Further Pure Endothermic Reaction With Equation Because the heat is absorbed by the system, the \(177.8 \: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The reaction between ethanoic acid and sodium carbonate. Some examples of endothermic reactions are: The. Endothermic Reaction With Equation.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Reaction With Equation The absorbed energy provides the activation energy for the reaction to. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. Of calcium carbonate in a blast furnace. \text{kj}\) is written as a reactant. An endothermic. Endothermic Reaction With Equation.
From www.teachoo.com
Chemical Equation Meaning, How to Write [with 5+ Examples] Teachoo Endothermic Reaction With Equation \text{mol}\) of calcium oxide and \(1 \: The reaction is between hydrogen ions from the acid and hydrogencarbonate ions. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The absorbed energy provides the activation energy for the reaction to. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. Endothermic Reaction With Equation.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction With Equation Some examples of endothermic reactions are: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. \text{kj}\) of heat is absorbed. The δh is positive for an endothermic. Of calcium carbonate in a blast furnace. Because the heat is absorbed by the system, the \(177.8 \: The heat is absorbed. Endothermic Reaction With Equation.
From ar.inspiredpencil.com
Endothermic Reaction Equation Endothermic Reaction With Equation An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. \text{mol}\) of calcium oxide and \(1 \: The reaction between ethanoic acid and sodium carbonate. \text{mol}\) of calcium carbonate decomposes into \(1 \: The heat is absorbed from the surroundings allowing the reactants to. For an endothermic chemical reaction to proceed, the reactants must absorb energy. Endothermic Reaction With Equation.
From narodnatribuna.info
Endothermic Reaction Examples With Equations Lorecentral Endothermic Reaction With Equation Some examples of endothermic reactions are: \text{kj}\) is written as a reactant. \text{mol}\) of calcium oxide and \(1 \: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. \text{mol}\) of calcium carbonate decomposes into \(1 \: The absorbed energy provides the activation energy for the reaction to. A chemical. Endothermic Reaction With Equation.
From ar.inspiredpencil.com
Endothermic Reaction Equation Endothermic Reaction With Equation For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is any chemical reaction that absorbs heat from its environment. \text{mol}\) of calcium oxide and \(1 \: The absorbed energy provides the activation energy for the reaction to. The reaction between ethanoic acid and sodium carbonate. The. Endothermic Reaction With Equation.
From www.youtube.com
Chemical Reaction and equation (Exothermic and Endothermic Reaction) class 10th science YouTube Endothermic Reaction With Equation The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because the heat is absorbed by the system, the \(177.8 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \: Of calcium carbonate in a blast furnace. Some examples of endothermic reactions. Endothermic Reaction With Equation.
From studymind.co.uk
Endothermic vs Exothermic Reactions (GCSE Chemistry) Study Mind Endothermic Reaction With Equation An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Of calcium carbonate in a blast furnace. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The heat is absorbed from the surroundings allowing the reactants to. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The. Endothermic Reaction With Equation.
From www.youtube.com
Endothermic or exothermic chemical equation YouTube Endothermic Reaction With Equation A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. Of calcium carbonate in a blast furnace. The δh is positive for an endothermic. The absorbed energy provides the activation energy for the reaction to. The heat is absorbed from the surroundings allowing the reactants to. \text{mol}\) of carbon dioxide, \(177.8 \: For an endothermic chemical. Endothermic Reaction With Equation.
From www.youtube.com
Endothermic & Exothermic reaction class 10 chemical reaction and equation YouTube Endothermic Reaction With Equation An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples of endothermic reactions are: The heat is absorbed from the surroundings allowing the reactants to. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is any chemical reaction that absorbs heat from its environment. For an endothermic chemical reaction to proceed, the. Endothermic Reaction With Equation.