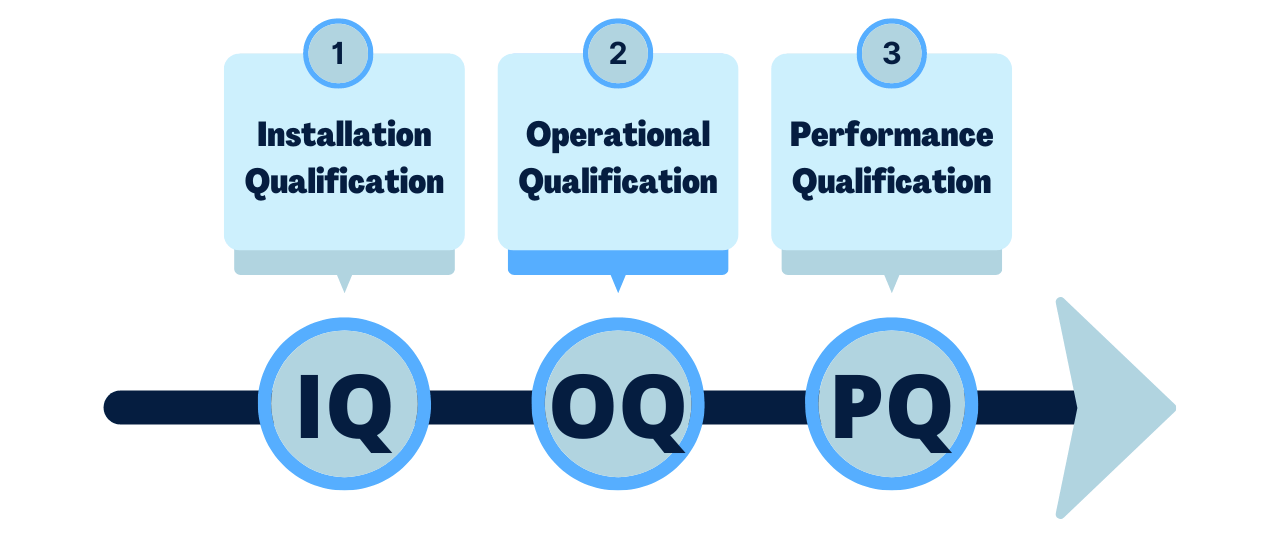
Medical Device Validation Definition . in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. It is usually done by. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. understanding iq, oq, and pq for medical device manufacturing processes. explains how precise verification and validation of medical devices help with approval and audits. The goal of process validation. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical.
from fasttrackiso13485.com
in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. explains how precise verification and validation of medical devices help with approval and audits. It is usually done by. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. understanding iq, oq, and pq for medical device manufacturing processes. The goal of process validation.
Fast Track ISO 13485 Process Validation Explained for your Medical Device
Medical Device Validation Definition explains how precise verification and validation of medical devices help with approval and audits. It is usually done by. understanding iq, oq, and pq for medical device manufacturing processes. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. The goal of process validation. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. explains how precise verification and validation of medical devices help with approval and audits.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Validation Definition validation is the process of making sure that you have objective evidence that user needs and intended uses are met. It is usually done by. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. understanding iq, oq, and pq for medical device manufacturing. Medical Device Validation Definition.
From www.qualitymeddev.com
Process Validation for Medical Devices Overview of FDA Requirements Medical Device Validation Definition explains how precise verification and validation of medical devices help with approval and audits. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. The goal of process validation. It is usually done by. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical. Medical Device Validation Definition.
From www.presentationeze.com
Medical Device ValidationPresentationEZE Medical Device Validation Definition learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. validation is the process of making sure that you have objective evidence that user needs and intended. Medical Device Validation Definition.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Validation Definition learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. understanding iq, oq, and pq for medical device manufacturing processes. It is usually done by. explains how precise verification and validation of medical devices help with approval and audits. in practice, sponsors of. Medical Device Validation Definition.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Validation Definition It is usually done by. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. understanding iq, oq, and pq for medical device manufacturing processes. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for. Medical Device Validation Definition.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Medical Device Validation Definition understanding iq, oq, and pq for medical device manufacturing processes. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. validation is the process of making. Medical Device Validation Definition.
From swarali-bhakre.medium.com
Process Validation for medical devices by IZiel Healthcare Medium Medical Device Validation Definition learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. The goal of process validation. understanding iq, oq, and pq for medical device manufacturing processes. explains how precise verification and validation of medical devices help with approval and audits. in practice, sponsors of. Medical Device Validation Definition.
From www.sifo-medical.com
Test Method Validation (TMV) in Medical Device Manufacturing Medical Device Validation Definition understanding iq, oq, and pq for medical device manufacturing processes. The goal of process validation. It is usually done by. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. explains how precise verification and validation of medical devices help with approval and audits.. Medical Device Validation Definition.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Validation Definition The goal of process validation. It is usually done by. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. explains how precise verification and validation of medical devices help with approval and audits. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical. Medical Device Validation Definition.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Validation Definition learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. The goal of process validation. explains how precise verification and validation of medical devices help with approval and audits. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will. Medical Device Validation Definition.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Validation Definition explains how precise verification and validation of medical devices help with approval and audits. The goal of process validation. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. understanding iq, oq, and pq for medical device manufacturing processes. in practice, sponsors of new medical products. Medical Device Validation Definition.
From tsquality.ch
The Future of Validation & Verification in Medical Devices Industry Medical Device Validation Definition explains how precise verification and validation of medical devices help with approval and audits. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical.. Medical Device Validation Definition.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Medical Device Validation Definition understanding iq, oq, and pq for medical device manufacturing processes. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. explains how precise verification and validation of medical devices. Medical Device Validation Definition.
From www.youtube.com
Process Validation Principles and Protocols for Medical Devices YouTube Medical Device Validation Definition validation is the process of making sure that you have objective evidence that user needs and intended uses are met. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. The goal of process validation. understanding iq, oq, and pq for medical device manufacturing processes. explains how precise verification. Medical Device Validation Definition.
From www.youtube.com
Difference between Verification and Validation ISO 9001 Definitions Medical Device Validation Definition It is usually done by. The goal of process validation. understanding iq, oq, and pq for medical device manufacturing processes. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. validation is the process of making sure that you have objective evidence that user. Medical Device Validation Definition.
From www.scribd.com
Validation & Qualification of Medical Device PDF Verification And Medical Device Validation Definition understanding iq, oq, and pq for medical device manufacturing processes. explains how precise verification and validation of medical devices help with approval and audits. It is usually done by. The goal of process validation. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical.. Medical Device Validation Definition.
From www.presentationeze.com
FDA Validation Requirements for Medical Devices PresentationEZE Medical Device Validation Definition It is usually done by. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. The goal of process validation. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. explains how precise verification. Medical Device Validation Definition.
From www.slideshare.net
Effective Medical Device Validation Introduction 2 Medical Device Validation Definition The goal of process validation. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. validation is the process of making sure that you have objective evidence. Medical Device Validation Definition.
From www.slideshare.net
Effective Medical Device Validation Introduction 2 Medical Device Validation Definition explains how precise verification and validation of medical devices help with approval and audits. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. understanding iq, oq, and pq for medical device manufacturing processes. It is usually done by. The goal of process validation. learn exactly what design verification. Medical Device Validation Definition.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Validation Definition validation is the process of making sure that you have objective evidence that user needs and intended uses are met. It is usually done by. understanding iq, oq, and pq for medical device manufacturing processes. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. The goal of process validation.. Medical Device Validation Definition.
From www.slideshare.net
Effective medical device validation introduction manual advance Medical Device Validation Definition It is usually done by. explains how precise verification and validation of medical devices help with approval and audits. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. The goal of process validation. in practice, sponsors of new medical products (drugs, biologics, or. Medical Device Validation Definition.
From www.presentationeze.com
Medical Device Validation PresentationEZE Medical Device Validation Definition learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. explains how precise verification and validation of medical devices help with approval and audits. understanding iq, oq, and pq for medical device manufacturing processes. in practice, sponsors of new medical products (drugs, biologics,. Medical Device Validation Definition.
From www.presentationeze.com
Medical Device Validation Requirements Principles & Practices Medical Device Validation Definition learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers. Medical Device Validation Definition.
From www.omnica.com
Medical Device Verification and Validation Omnica Corporation Medical Device Validation Definition validation is the process of making sure that you have objective evidence that user needs and intended uses are met. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. understanding iq, oq, and pq for medical device manufacturing processes. It is usually done by. The goal of process validation.. Medical Device Validation Definition.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V Medical Device Validation Definition in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. It is usually done by. explains how precise verification and validation of medical devices help with approval and audits. The goal of process validation. learn exactly what design verification and design validation are, how they are the same, how they. Medical Device Validation Definition.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Validation Definition The goal of process validation. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. explains how precise verification and validation of medical devices help with approval and audits. It is usually done by. understanding iq, oq, and pq for medical device manufacturing processes. learn exactly what design verification. Medical Device Validation Definition.
From www.ideagen.com
What Is Medical Device Validation? Medical Device Validation Definition It is usually done by. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. in practice, sponsors of new medical products (drugs, biologics,. Medical Device Validation Definition.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Medical Device Validation Definition learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. understanding iq, oq, and pq for medical device manufacturing processes. The goal of process validation. It is usually done by. explains how precise verification and validation of medical devices help with approval and audits.. Medical Device Validation Definition.
From www.presentationeze.com
Medical Device ValidationPresentationEZE Medical Device Validation Definition learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. It is usually done by. validation is the process of making sure that you have objective evidence. Medical Device Validation Definition.
From www.youtube.com
What is Medical Device Process Validation? Operon Strategist YouTube Medical Device Validation Definition understanding iq, oq, and pq for medical device manufacturing processes. explains how precise verification and validation of medical devices help with approval and audits. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. It is usually done by. in practice, sponsors of new medical products. Medical Device Validation Definition.
From www.joharidigital.com
The Ultimate guide to Validation & Verification of Medical Devices Medical Device Validation Definition validation is the process of making sure that you have objective evidence that user needs and intended uses are met. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices for medical. It is usually done by. The goal of process validation. explains how precise verification. Medical Device Validation Definition.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Validation Definition explains how precise verification and validation of medical devices help with approval and audits. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. The goal of process validation. learn exactly what design verification and design validation are, how they are the same, how they are different, and best practices. Medical Device Validation Definition.
From www.slideshare.net
Process Validation for Medical Device Compliance Medical Device Validation Definition understanding iq, oq, and pq for medical device manufacturing processes. validation is the process of making sure that you have objective evidence that user needs and intended uses are met. It is usually done by. explains how precise verification and validation of medical devices help with approval and audits. The goal of process validation. learn exactly. Medical Device Validation Definition.
From arrotek.com
Medical Device Design Validation Explained Arrotek Medical Device Validation Definition understanding iq, oq, and pq for medical device manufacturing processes. It is usually done by. The goal of process validation. explains how precise verification and validation of medical devices help with approval and audits. in practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the. learn exactly what design verification. Medical Device Validation Definition.