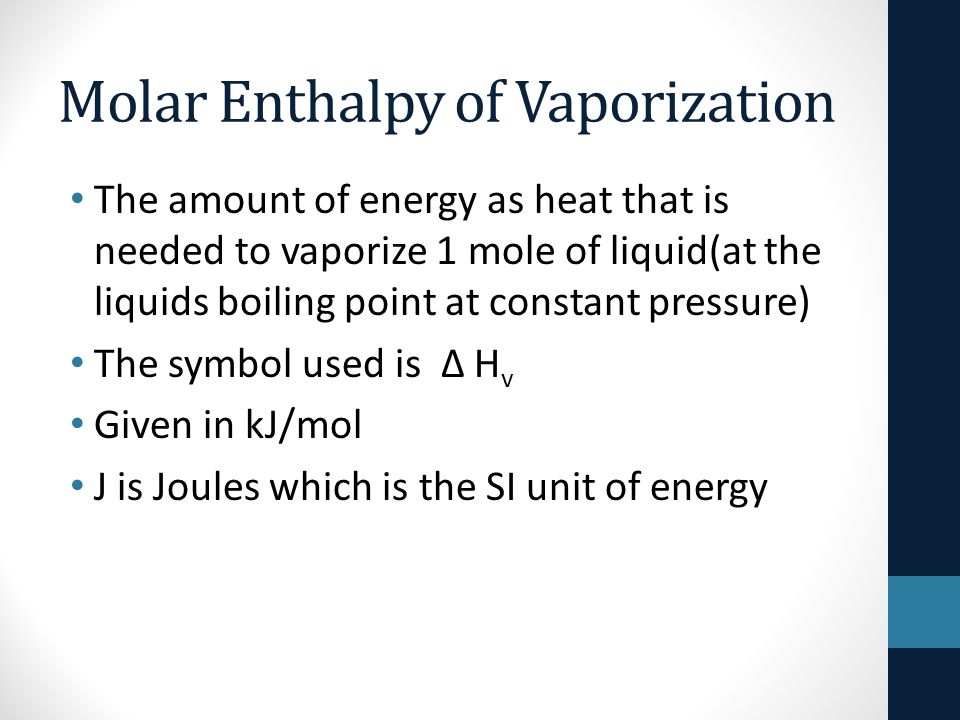
Standard Molar Heat Vaporization . The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the molar heat of vaporization equation looks like this: the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. Q = (δh vap) (mass/molar mass) the meanings are as follows:
from slidesharetrick.blogspot.com
Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. Q = (δh vap) (mass/molar mass) the meanings are as follows: the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the molar heat of vaporization equation looks like this: the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that.
Molar Enthalpy Of Vaporization Calculator slidesharetrick
Standard Molar Heat Vaporization Q = (δh vap) (mass/molar mass) the meanings are as follows: Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. Q = (δh vap) (mass/molar mass) the meanings are as follows: The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the molar heat of vaporization equation looks like this: the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid.
From www.chegg.com
Solved Calculate the standard molar entropy of vaporization Standard Molar Heat Vaporization the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually. Standard Molar Heat Vaporization.
From www.chegg.com
Solved Calculate the standard molar entropy of vaporization Standard Molar Heat Vaporization the molar heat of vaporization equation looks like this: Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. Q = (δh vap) (mass/molar mass) the meanings are as follows: the heat which. Standard Molar Heat Vaporization.
From slideplayer.com
Chapter ppt download Standard Molar Heat Vaporization Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that.. Standard Molar Heat Vaporization.
From www.youtube.com
Heat of Vaporization from Vapor Pressure (Example) YouTube Standard Molar Heat Vaporization The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. Q = (δh vap) (mass/molar mass) the meanings are as follows: the molar. Standard Molar Heat Vaporization.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Standard Molar Heat Vaporization the molar heat of vaporization equation looks like this: Q = (δh vap) (mass/molar mass) the meanings are as follows: The units are usually kilojoules per mole, or kj/mol. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. the molar heat of. Standard Molar Heat Vaporization.
From www.researchgate.net
Standard molar enthalpy, entropy and heat capacity of hydration of Standard Molar Heat Vaporization the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the molar heat of vaporization equation looks like. Standard Molar Heat Vaporization.
From www.slideserve.com
PPT Chapter 17 The Direction of Chemical Change (The Second Law of Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the molar heat of vaporization equation looks like this: Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the molar heat of vaporization is the energy needed. Standard Molar Heat Vaporization.
From www.slideserve.com
PPT How heat Is Measured PowerPoint Presentation, free download ID Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. Q = (δh vap) (mass/molar mass) the meanings are as follows: The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization equation looks like this: Values refer to the enthalpy change in the. Standard Molar Heat Vaporization.
From www.numerade.com
SOLVEDUse the following standard heats of formation to calculate the Standard Molar Heat Vaporization Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the molar heat of vaporization equation looks like this: Q = (δh vap) (mass/molar mass) the meanings are as follows: the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and. Standard Molar Heat Vaporization.
From www.chegg.com
Solved Calculate the standard molar entropy of vaporization Standard Molar Heat Vaporization The units are usually kilojoules per mole, or kj/mol. Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the molar heat of vaporization equation looks like this: the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the molar. Standard Molar Heat Vaporization.
From www.studyxapp.com
1 calculate the standard molar entropy of vaporization of water at 360 Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. Q = (δh vap) (mass/molar mass) the meanings are as follows: the. Standard Molar Heat Vaporization.
From www.slideshare.net
Enthalpy of vaporization of liquid Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. The units are usually kilojoules per mole, or kj/mol. Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. Q = (δh vap) (mass/molar mass) the meanings are as follows:. Standard Molar Heat Vaporization.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID3528115 Standard Molar Heat Vaporization the molar heat of vaporization equation looks like this: Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. The units are usually kilojoules per mole,. Standard Molar Heat Vaporization.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3206797 Standard Molar Heat Vaporization Q = (δh vap) (mass/molar mass) the meanings are as follows: the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. the heat of vaporization (also called. Standard Molar Heat Vaporization.
From www.numerade.com
SOLVEDThe standard enthalpy of formation of gaseous molecular bromine Standard Molar Heat Vaporization the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. The units are usually kilojoules per mole, or kj/mol. Values refer to the enthalpy change in the conversion. Standard Molar Heat Vaporization.
From www.numerade.com
SOLVED 4 Calculate the standard molar entropy of vaporization of Standard Molar Heat Vaporization Q = (δh vap) (mass/molar mass) the meanings are as follows: the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat which a solid absorbs when it melts is called. Standard Molar Heat Vaporization.
From www.chegg.com
Solved Calculate the standard molar entropy of vaporization Standard Molar Heat Vaporization the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. The units are usually kilojoules per mole, or kj/mol. Q = (δh vap) (mass/molar mass) the meanings are as follows: the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole. Standard Molar Heat Vaporization.
From www.youtube.com
Molar Enthalpy Calculations YouTube Standard Molar Heat Vaporization the molar heat of vaporization equation looks like this: the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. Q = (δh vap) (mass/molar mass) the meanings. Standard Molar Heat Vaporization.
From www.slideserve.com
PPT OBJECTIVES PowerPoint Presentation, free download ID3301763 Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. Q = (δh vap) (mass/molar mass) the meanings are as follows: the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. Values refer to the enthalpy change in the conversion of. Standard Molar Heat Vaporization.
From www.numerade.com
SOLVEDThe standard molar entropy of iodine vapor, I2( g), is 260.7 J K Standard Molar Heat Vaporization the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. The units are usually kilojoules per mole, or kj/mol. Q = (δh vap) (mass/molar mass) the meanings are as follows: Values refer to the enthalpy change in the conversion of liquid to gas at the. Standard Molar Heat Vaporization.
From www.chegg.com
Solved Calculate the standard molar entropy of vaporization Standard Molar Heat Vaporization The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. Values refer to the enthalpy change in the conversion of liquid to. Standard Molar Heat Vaporization.
From slidesharetrick.blogspot.com
Molar Enthalpy Of Vaporization Calculator slidesharetrick Standard Molar Heat Vaporization the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and. Standard Molar Heat Vaporization.
From slideplayer.com
Heat, what does it really mean? ppt download Standard Molar Heat Vaporization The units are usually kilojoules per mole, or kj/mol. Q = (δh vap) (mass/molar mass) the meanings are as follows: the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. the heat of vaporization (also called the enthalpy of vaporization) is the heat required. Standard Molar Heat Vaporization.
From www.chegg.com
Solved Calculate the standard molar entropy of vaporization Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the molar heat of vaporization equation looks like this: Q = (δh vap) (mass/molar mass) the meanings are as follows: Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point. Standard Molar Heat Vaporization.
From www.numerade.com
SOLVEDUse the following standard heats of formation to calculate the Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and. Standard Molar Heat Vaporization.
From studylib.net
Molar Heat of Fusion and Molar Heat of Vaporization Worksheet Standard Molar Heat Vaporization The units are usually kilojoules per mole, or kj/mol. Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the heat which a solid absorbs when it melts is called the. Standard Molar Heat Vaporization.
From ar.inspiredpencil.com
Molar Enthalpy Of Vaporization Examples Standard Molar Heat Vaporization the molar heat of vaporization equation looks like this: the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. Values refer to the enthalpy change in the conversion of liquid to gas. Standard Molar Heat Vaporization.
From ar.inspiredpencil.com
Molar Enthalpy Of Vaporization Examples Standard Molar Heat Vaporization The units are usually kilojoules per mole, or kj/mol. Q = (δh vap) (mass/molar mass) the meanings are as follows: Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that.. Standard Molar Heat Vaporization.
From www.ck12.org
Heats of Vaporization and Condensation CK12 Foundation Standard Molar Heat Vaporization the molar heat of vaporization equation looks like this: the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. The units are usually kilojoules per mole, or kj/mol. Q = (δh vap) (mass/molar mass) the meanings are as follows: the molar heat of. Standard Molar Heat Vaporization.
From www.slideserve.com
PPT Heat & Changes of State PowerPoint Presentation, free download Standard Molar Heat Vaporization the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization \(\left( \delta. Standard Molar Heat Vaporization.
From ar.inspiredpencil.com
Molar Enthalpy Of Vaporization Standard Molar Heat Vaporization The units are usually kilojoules per mole, or kj/mol. the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this phase change. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the molar heat of vaporization equation looks like. Standard Molar Heat Vaporization.
From www.chegg.com
Solved Estimate the standard molar enthalpy of vaporization Standard Molar Heat Vaporization the molar heat of vaporization equation looks like this: Values refer to the enthalpy change in the conversion of liquid to gas at the boiling point (normal,. The units are usually kilojoules per mole, or kj/mol. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat of vaporization (also. Standard Molar Heat Vaporization.
From www.numerade.com
SOLVED The standard molar enthalpy of vaporization for acetone is +31. Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the molar heat of vaporization equation looks like this: the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat which a solid absorbs when it melts is. Standard Molar Heat Vaporization.
From www.pinterest.com
Molar Enthalpy Of Vaporization Easy Science Study chemistry Standard Molar Heat Vaporization the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. the molar heat of vaporization is the energy needed to vaporize one mole of a liquid. the heat of vaporization (also called the enthalpy of vaporization) is the heat required to induce this. Standard Molar Heat Vaporization.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3206797 Standard Molar Heat Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. The units are usually kilojoules per mole, or kj/mol. Q = (δh vap) (mass/molar mass) the meanings are as follows: the heat which a solid absorbs when it melts is called the enthalpy of fusion or heat. Standard Molar Heat Vaporization.