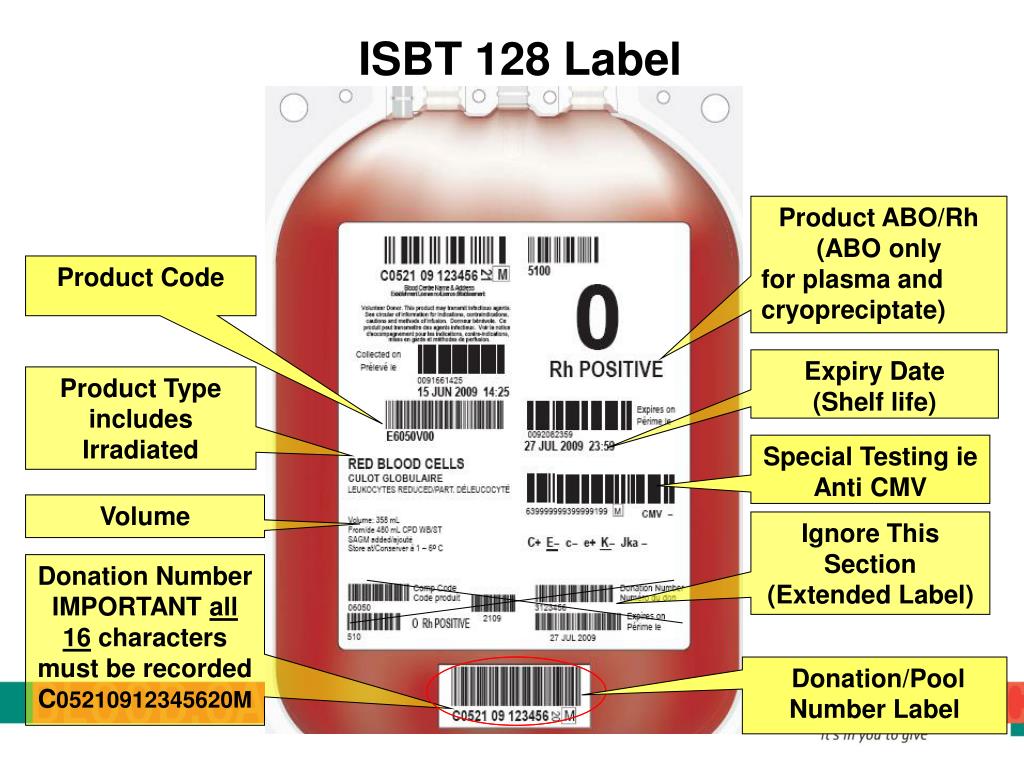
Blood Bank Unit Labels . Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating to the donor, a product code,. By the fda in a guidance dated september 22, 2006,. This format, approved for use in the u.s. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products). 5/5 (256) Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. It was developed as an extension of. A specific format, known as isbt 128, is required. Circular of information for the use of human blood and blood components is considered to be labeling. Patient safety starts with positive identification. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. The label applied to the blood pack by the. The labelling system for blood and blood components comprises the following elements:
from www.slideserve.com
Patient safety starts with positive identification. It was developed as an extension of. 5/5 (256) Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating to the donor, a product code,. The labelling system for blood and blood components comprises the following elements: Circular of information for the use of human blood and blood components is considered to be labeling. By the fda in a guidance dated september 22, 2006,. The label applied to the blood pack by the. This format, approved for use in the u.s. A specific format, known as isbt 128, is required.
PPT ISBT 128 Implementation Changes to Blood Component Labels Spring
Blood Bank Unit Labels Circular of information for the use of human blood and blood components is considered to be labeling. This format, approved for use in the u.s. The label applied to the blood pack by the. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products). A specific format, known as isbt 128, is required. 5/5 (256) Circular of information for the use of human blood and blood components is considered to be labeling. Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating to the donor, a product code,. It was developed as an extension of. By the fda in a guidance dated september 22, 2006,. The labelling system for blood and blood components comprises the following elements: Patient safety starts with positive identification. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment.
From www.barcodefactory.com
Blood and Plasma Bag Labels BarcodeFactory Blood Bank Unit Labels A specific format, known as isbt 128, is required. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. 5/5 (256) Patient safety starts with positive identification. The labelling system for blood and blood components comprises the following elements: Each label must have information that contains, at a minimum, a unique facility identifier, a lot. Blood Bank Unit Labels.
From www.mercianlabels.com
Blood Bank Labels Mercian Labels Blood Bank Unit Labels Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products). The labelling system for blood and blood components comprises the following elements: This format, approved for use in the u.s. 5/5 (256) The label applied to the blood pack by the. It. Blood Bank Unit Labels.
From www.optimalblooduse.eu
6.2 Blood component label Optimal Blood Use Blood Bank Unit Labels A specific format, known as isbt 128, is required. Circular of information for the use of human blood and blood components is considered to be labeling. This format, approved for use in the u.s. 5/5 (256) Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Each label must have information that contains, at a. Blood Bank Unit Labels.
From trauma-news.com
Setting up a whole blood program? 5 questions you need to answer Blood Bank Unit Labels A specific format, known as isbt 128, is required. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products. Blood Bank Unit Labels.
From www.labelvalue.com
Blood Bank Medical Labels Free Shipping Blood Bank Unit Labels Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. 5/5 (256) Circular of information for the use of human blood and blood components is considered to be labeling. Each label must have information. Blood Bank Unit Labels.
From www.transfusionguidelines.org
Blood group labels Blood Bank Unit Labels 5/5 (256) It was developed as an extension of. The labelling system for blood and blood components comprises the following elements: Circular of information for the use of human blood and blood components is considered to be labeling. By the fda in a guidance dated september 22, 2006,. Representatives from international blood banking organizations contributed over many years to. Blood Bank Unit Labels.
From www.wlp.com
Pre Printed Blood Bag Labels & ISBT 128 Barcodes Watson Label Blood Bank Unit Labels By the fda in a guidance dated september 22, 2006,. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. This format, approved for use in the u.s. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood,. Blood Bank Unit Labels.
From www.myfisherstore.com
Blood Banking and Large Capacity Centrifugation Blood Bank Unit Labels The label applied to the blood pack by the. The labelling system for blood and blood components comprises the following elements: Circular of information for the use of human blood and blood components is considered to be labeling. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. It. Blood Bank Unit Labels.
From www.upmraflatac.com
IIMAK Keeping patients safe with durable blood bag labels UPM Raflatac Blood Bank Unit Labels The label applied to the blood pack by the. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products. Blood Bank Unit Labels.
From www.dreamstime.com
Blood Bag with Label Realistic Vector Illustration Stock Vector Blood Bank Unit Labels The label applied to the blood pack by the. Circular of information for the use of human blood and blood components is considered to be labeling. Patient safety starts with positive identification. The labelling system for blood and blood components comprises the following elements: By the fda in a guidance dated september 22, 2006,. Representatives from international blood banking organizations. Blood Bank Unit Labels.
From templates.rjuuc.edu.np
Blood Transfusion Blood Bag Label Template Blood Bank Unit Labels The labelling system for blood and blood components comprises the following elements: Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. By the fda in a guidance dated september 22, 2006,. Patient safety starts with positive identification. Each label must have information that contains, at a minimum, a. Blood Bank Unit Labels.
From www.xinxinglabel.com
Blood Bag Label Professional Label Printing Manufacturer Blood Bank Unit Labels 5/5 (256) Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. Circular of information for the use of human blood and blood components is considered to be labeling. The labelling system for blood and blood components comprises the following elements: It was developed as an extension of.. Blood Bank Unit Labels.
From www.digi-trax.com
Four Quadrant ISBT 128 Blood Bank Label DigiTrax® Blood Bank Unit Labels This format, approved for use in the u.s. It was developed as an extension of. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products). Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating. Blood Bank Unit Labels.
From teachmephysiology.com
Blood Groups Rhesus positive ABO grouping TeachMePhysiology Blood Bank Unit Labels By the fda in a guidance dated september 22, 2006,. The label applied to the blood pack by the. This format, approved for use in the u.s. Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating to the donor, a product code,. It was developed as an extension of. Patient safety. Blood Bank Unit Labels.
From dl-uk.apowersoft.com
Blood Bag Label Template Blood Bank Unit Labels Patient safety starts with positive identification. Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating to the donor, a product code,. The label applied to the blood pack by the. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable.. Blood Bank Unit Labels.
From www.slideserve.com
PPT ISBT 128 Blood Product Labeling for Transfusionists Americas Blood Bank Unit Labels A specific format, known as isbt 128, is required. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Circular of information for the use of human blood and blood components is considered to be labeling. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are. Blood Bank Unit Labels.
From info.weberpackaging.com
Blood & IV Bag Labeling Solutions Blood Bank Unit Labels The label applied to the blood pack by the. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products). Circular of information for the use of human blood and blood components is considered to be labeling. A specific format, known as isbt 128,. Blood Bank Unit Labels.
From maysamustafa.blogspot.com
Blood Transfusion Blood Bag Label Template Barcode Specifications Blood Bank Unit Labels The label applied to the blood pack by the. 5/5 (256) Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products). This format, approved for use in the. Blood Bank Unit Labels.
From www.transfusionguidelines.org
Blood group labels Blood Bank Unit Labels Circular of information for the use of human blood and blood components is considered to be labeling. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. By the fda in a guidance dated september 22, 2006,. Patient safety starts with positive identification. Each label must have information that contains, at a minimum, a unique facility. Blood Bank Unit Labels.
From www.optimalblooduse.eu
6.2 Blood component label Optimal Blood Use Blood Bank Unit Labels It was developed as an extension of. Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating to the donor, a product code,. The labelling system for blood and blood components comprises the following elements: A specific format, known as isbt 128, is required. The label applied to the blood pack by. Blood Bank Unit Labels.
From hagueaustralia.com.au
Trace Safe™ Blood Labelling Hague Australia Blood Bank Unit Labels Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products). A specific format, known as isbt 128, is required. The labelling system for blood and blood components comprises the following elements: Each label must have information that contains, at a minimum, a unique. Blood Bank Unit Labels.
From www.alamy.com
unit of blood for transfusion Stock Photo Alamy Blood Bank Unit Labels 5/5 (256) The label applied to the blood pack by the. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products).. Blood Bank Unit Labels.
From profedu.blood.ca
Whole blood donors with antibodies Professional Education Blood Bank Unit Labels Patient safety starts with positive identification. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. The label applied to the blood pack by the. Circular of information for the use of human blood and blood components is considered to be labeling. The labelling system for blood and blood components comprises the following elements: Each label. Blood Bank Unit Labels.
From www.slideserve.com
PPT ISBT 128 Implementation Changes to Blood Component Labels Spring Blood Bank Unit Labels Circular of information for the use of human blood and blood components is considered to be labeling. By the fda in a guidance dated september 22, 2006,. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that. Blood Bank Unit Labels.
From www.alamy.com
Unit stored blood, erythrocyte concentrate, blood, blood bank, blood Blood Bank Unit Labels This format, approved for use in the u.s. Patient safety starts with positive identification. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. Circular of information for the use of human blood and blood components is considered to be labeling. Isbt 128 is the global standard for the. Blood Bank Unit Labels.
From blood.ca
Label Format and Material Canadian Blood Services Blood Bank Unit Labels Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. It was developed as an extension of. This format, approved for use in the u.s. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk,. Blood Bank Unit Labels.
From www.etsy.com
8 Blood Labels Easy Editable DIY Home Printable Digital Etsy Sweden Blood Bank Unit Labels Circular of information for the use of human blood and blood components is considered to be labeling. This format, approved for use in the u.s. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating to the donor,. Blood Bank Unit Labels.
From label.averydennison.com
Blood Bag Labels Avery Dennison LPM Blood Bank Unit Labels Each label must have information that contains, at a minimum, a unique facility identifier, a lot number relating to the donor, a product code,. A specific format, known as isbt 128, is required. It was developed as an extension of. By the fda in a guidance dated september 22, 2006,. Pdc laboratory labels are high quality, built for use in. Blood Bank Unit Labels.
From www.labtag.com
Blood Bag Secondary MultiPart Labels 3.875″ x 3.875" HFJT296 Blood Bank Unit Labels 5/5 (256) Patient safety starts with positive identification. The labelling system for blood and blood components comprises the following elements: The label applied to the blood pack by the. It was developed as an extension of. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. This format,. Blood Bank Unit Labels.
From www.healthysimulation.com
Blood Transfusion Documents and Excel Labels for Simulation in Blood Bank Unit Labels Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products). This format, approved for use in the u.s. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. Each label must have. Blood Bank Unit Labels.
From www.geocities.ws
Free Blood Bank Component Labels Blood Bank Unit Labels Circular of information for the use of human blood and blood components is considered to be labeling. This format, approved for use in the u.s. The label applied to the blood pack by the. Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. Each label must have information. Blood Bank Unit Labels.
From transfusion.com.au
Blood component Codabar label Australian Red Cross Lifeblood Blood Bank Unit Labels The label applied to the blood pack by the. By the fda in a guidance dated september 22, 2006,. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin (including blood, cell, tissue, milk, and organ products).. Blood Bank Unit Labels.
From www.shamrocklabels.com
Blood Bank Labels Blood Bag Label Adhesives Shamrock Labels Blood Bank Unit Labels It was developed as an extension of. This format, approved for use in the u.s. Circular of information for the use of human blood and blood components is considered to be labeling. Pdc laboratory labels are high quality, built for use in the demanding healthcare environment. The label applied to the blood pack by the. 5/5 (256) Representatives from. Blood Bank Unit Labels.
From www.wlp.com
Pre Printed Blood Bag Labels & ISBT 128 Barcodes Watson Label Blood Bank Unit Labels 5/5 (256) The labelling system for blood and blood components comprises the following elements: The label applied to the blood pack by the. Circular of information for the use of human blood and blood components is considered to be labeling. Isbt 128 is the global standard for the terminology, identification, coding and labeling of medical products of human origin. Blood Bank Unit Labels.
From www.alamy.com
Unit stored blood, erythrocyte concentrate, detail, blood, blood bank Blood Bank Unit Labels Patient safety starts with positive identification. The labelling system for blood and blood components comprises the following elements: This format, approved for use in the u.s. 5/5 (256) Representatives from international blood banking organizations contributed over many years to try to create a set of standards that are achievable. A specific format, known as isbt 128, is required. It. Blood Bank Unit Labels.