Lead Carbonate Heated Equation . lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. C 2 h 2 o 8 pb 3. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. The general form of a. a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. After the reaction, a solid remains in the test tube, but the solid is yellow.
from www.chegg.com
C 2 h 2 o 8 pb 3. After the reaction, a solid remains in the test tube, but the solid is yellow. a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. The general form of a. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated.
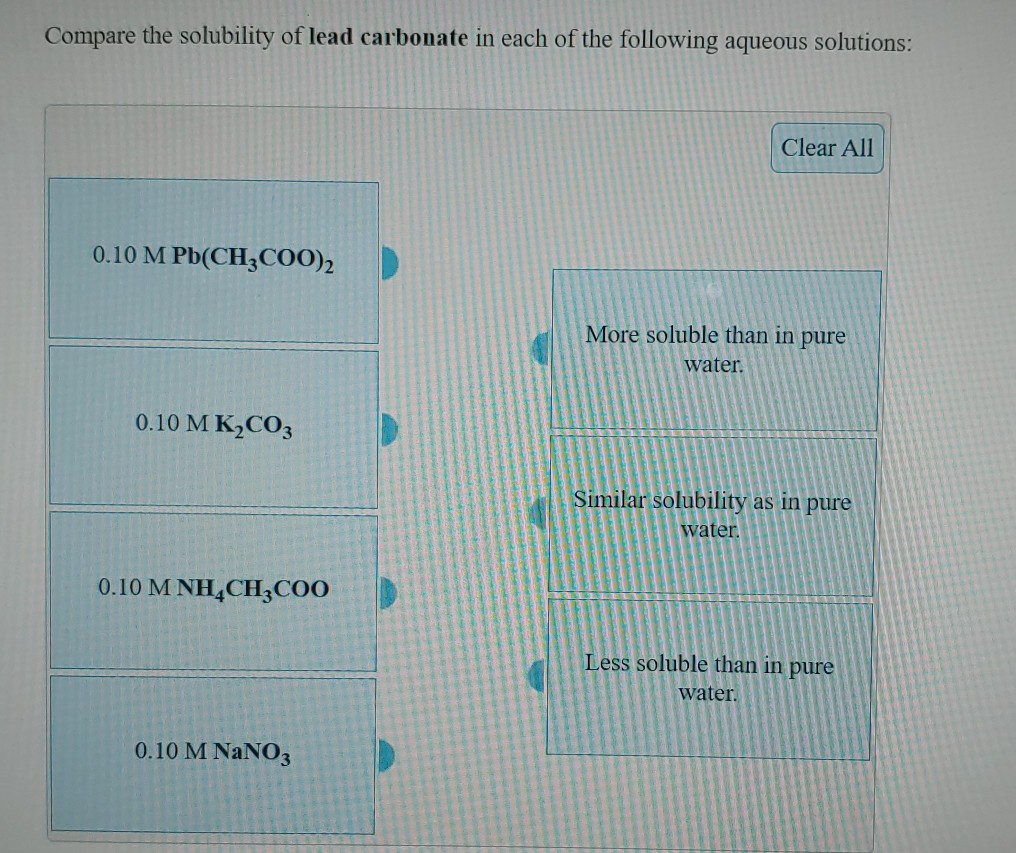
Solved Compare the solubility of lead carbonate in each of
Lead Carbonate Heated Equation revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. After the reaction, a solid remains in the test tube, but the solid is yellow. a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. The general form of a. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. C 2 h 2 o 8 pb 3.
From kunduz.com
[ANSWERED] Give the chemical formula for lead (IV) carbonate. Read Kunduz Lead Carbonate Heated Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. The general form of a. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. many metal carbonates are decomposed by heat. Lead Carbonate Heated Equation.
From www.numerade.com
SOLVED When 2.750 g of the lead oxide Pb3O4 is heated, it is Lead Carbonate Heated Equation revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. C 2 h 2 o 8 pb 3. The general form of a. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. Lead Carbonate Heated Equation.
From www.numerade.com
SOLVED Write a balance chemical equation for each of the following Lead Carbonate Heated Equation C 2 h 2 o 8 pb 3. a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. revision notes. Lead Carbonate Heated Equation.
From www.numerade.com
SOLVED The preparation of lead sulphate from lead carbonate is a two Lead Carbonate Heated Equation C 2 h 2 o 8 pb 3. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. After the. Lead Carbonate Heated Equation.
From www.youtube.com
How to write chemical formula of Lead CarbonateLead carbonate formula Lead Carbonate Heated Equation a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. After the reaction, a solid remains in the test tube, but. Lead Carbonate Heated Equation.
From www.numerade.com
SOLVEDWhen 2.750 g of the oxide of lead Pb3 O4 is strongly heated, it Lead Carbonate Heated Equation a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the. Lead Carbonate Heated Equation.
From www.fishersci.ca
Lead(II) carbonate, ACS, Thermo Scientific Chemicals Fisher Scientific Lead Carbonate Heated Equation a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. After the reaction, a solid remains in the test tube, but the solid is yellow. The general form of a. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals,. Lead Carbonate Heated Equation.
From brainly.in
write currently a balanced equation for the following word equation red Lead Carbonate Heated Equation lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. After the reaction, a solid remains in the test tube, but the solid is yellow. The general form of a. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save. Lead Carbonate Heated Equation.
From www.numerade.com
SOLVED The equation for the effect of heat on hydrated sodium Lead Carbonate Heated Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. C 2 h 2 o. Lead Carbonate Heated Equation.
From www.youtube.com
How to Balance Pb(NO3)2 = PbO + NO2 + O2 of Lead (II Lead Carbonate Heated Equation The general form of a. C 2 h 2 o 8 pb 3. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. After the reaction, a solid remains in the test tube, but the solid is yellow. revision. Lead Carbonate Heated Equation.
From www.youtube.com
How to write the equation for PbCO3 + H2O Lead (II) carbonate + Water Lead Carbonate Heated Equation a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a. C 2 h 2 o 8 pb 3. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. revision notes. Lead Carbonate Heated Equation.
From www.toppr.com
Complete the following table that refers to the action of heat on some Lead Carbonate Heated Equation After the reaction, a solid remains in the test tube, but the solid is yellow. C 2 h 2 o 8 pb 3. The general form of a. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. compare the thermal stabilities of carbonates. Lead Carbonate Heated Equation.
From www.youtube.com
How to Write the Formula for Lead (II) bicarbonate YouTube Lead Carbonate Heated Equation a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. C 2 h 2 o 8 pb 3. After the reaction, a solid remains in the test tube, but the solid is yellow. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is. Lead Carbonate Heated Equation.
From www.slideserve.com
PPT CHEMICALS IN ACTION PowerPoint Presentation, free download ID Lead Carbonate Heated Equation After the reaction, a solid remains in the test tube, but the solid is yellow. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. many metal. Lead Carbonate Heated Equation.
From www.youtube.com
Write correctly a balanced equation for the following word equation Lead Carbonate Heated Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. revision notes on thermal decomposition of metal carbonates for the oxford aqa. Lead Carbonate Heated Equation.
From www.youtube.com
Type of Reaction for PbCO3 = PbO + CO2 YouTube Lead Carbonate Heated Equation a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. C 2 h 2 o 8 pb 3. lead (ii) carbonate, which is a white. Lead Carbonate Heated Equation.
From studylib.net
File Lead Carbonate Heated Equation lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word. Lead Carbonate Heated Equation.
From www.numerade.com
Lead(II) oxide is obtained by roasting galena, lead(II) sulfide, in air Lead Carbonate Heated Equation a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. The general form of a. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the. Lead Carbonate Heated Equation.
From brainly.in
Write the balanced chemical equation of calcium carbonate on Lead Carbonate Heated Equation C 2 h 2 o 8 pb 3. After the reaction, a solid remains in the test tube, but the solid is yellow. The general form of a. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. lead (ii) carbonate, which is a. Lead Carbonate Heated Equation.
From brainly.in
give balanced equation for thermal of lead carbonate Lead Carbonate Heated Equation C 2 h 2 o 8 pb 3. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. a decomposition reaction is. Lead Carbonate Heated Equation.
From www.chegg.com
Solved Compare the solubility of lead carbonate in each of Lead Carbonate Heated Equation C 2 h 2 o 8 pb 3. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates. Lead Carbonate Heated Equation.
From studylib.net
Action of heat on transition metal carbonates In Lead Carbonate Heated Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. C 2 h 2 o 8 pb 3. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at. Lead Carbonate Heated Equation.
From www.numerade.com
Lead (II) carbonate to form lead (II) oxide and carbon Lead Carbonate Heated Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. revision notes on thermal decomposition of metal carbonates for the oxford aqa. Lead Carbonate Heated Equation.
From www.youtube.com
How to Write the Formula for Lead (II) carbonate YouTube Lead Carbonate Heated Equation revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. lead (ii) carbonate, which is a white solid, is. Lead Carbonate Heated Equation.
From www.youtube.com
How to write Molecular formula of lead oxide Chemical formula of Lead Lead Carbonate Heated Equation The general form of a. After the reaction, a solid remains in the test tube, but the solid is yellow. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. revision notes on thermal decomposition of metal carbonates for. Lead Carbonate Heated Equation.
From www.geological-digressions.com
Mineralogy of carbonates; basic geochemistry Geological Digressions Lead Carbonate Heated Equation After the reaction, a solid remains in the test tube, but the solid is yellow. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. C 2 h 2 o 8 pb 3. lead (ii) carbonate, which is a white solid, is the reactant. Lead Carbonate Heated Equation.
From www.numerade.com
SOLVED Lead metal is heated with oxygen in air to yield solid lead(IV Lead Carbonate Heated Equation After the reaction, a solid remains in the test tube, but the solid is yellow. a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal. Lead Carbonate Heated Equation.
From cekpqokm.blob.core.windows.net
Lead Carbonate Equation For Thermal at Kathleen Rosales blog Lead Carbonate Heated Equation many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. The general form of a. C 2 h 2 o 8 pb 3. revision notes on thermal. Lead Carbonate Heated Equation.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Lead Carbonate Heated Equation many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction. C 2 h 2 o 8 pb 3. revision notes on thermal decomposition of metal carbonates for. Lead Carbonate Heated Equation.
From www.numerade.com
SOLVED Part Write a balanced equation for the of lead Lead Carbonate Heated Equation The general form of a. C 2 h 2 o 8 pb 3. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. lead (ii) carbonate, which is a white solid, is the reactant of the thermal decomposition reaction.. Lead Carbonate Heated Equation.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Lead Carbonate Heated Equation After the reaction, a solid remains in the test tube, but the solid is yellow. The general form of a. a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the. Lead Carbonate Heated Equation.
From www.gauthmath.com
Solved Q2 A student heated solid lead carbonate, as shown in the Lead Carbonate Heated Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper metal carbonates decompose when heated. a decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. After the reaction, a solid remains in the test tube, but. Lead Carbonate Heated Equation.
From www.chegg.com
Solved 10 (a) The diagram shows the apparatus a teacher uses Lead Carbonate Heated Equation After the reaction, a solid remains in the test tube, but the solid is yellow. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive. Lead Carbonate Heated Equation.
From www.reddit.com
Converting standard to basic lead carbonate? chemhelp Lead Carbonate Heated Equation The general form of a. C 2 h 2 o 8 pb 3. revision notes on thermal decomposition of metal carbonates for the oxford aqa igcse chemistry syllabus, written by the chemistry experts at save my exams. After the reaction, a solid remains in the test tube, but the solid is yellow. lead (ii) carbonate, which is a. Lead Carbonate Heated Equation.
From www.slideserve.com
PPT What is a chemical reaction? PowerPoint Presentation, free Lead Carbonate Heated Equation many metal carbonates are decomposed by heat to form a metal oxide and carbon dioxide as is shown in the following general word equation. After the reaction, a solid remains in the test tube, but the solid is yellow. The general form of a. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and. Lead Carbonate Heated Equation.