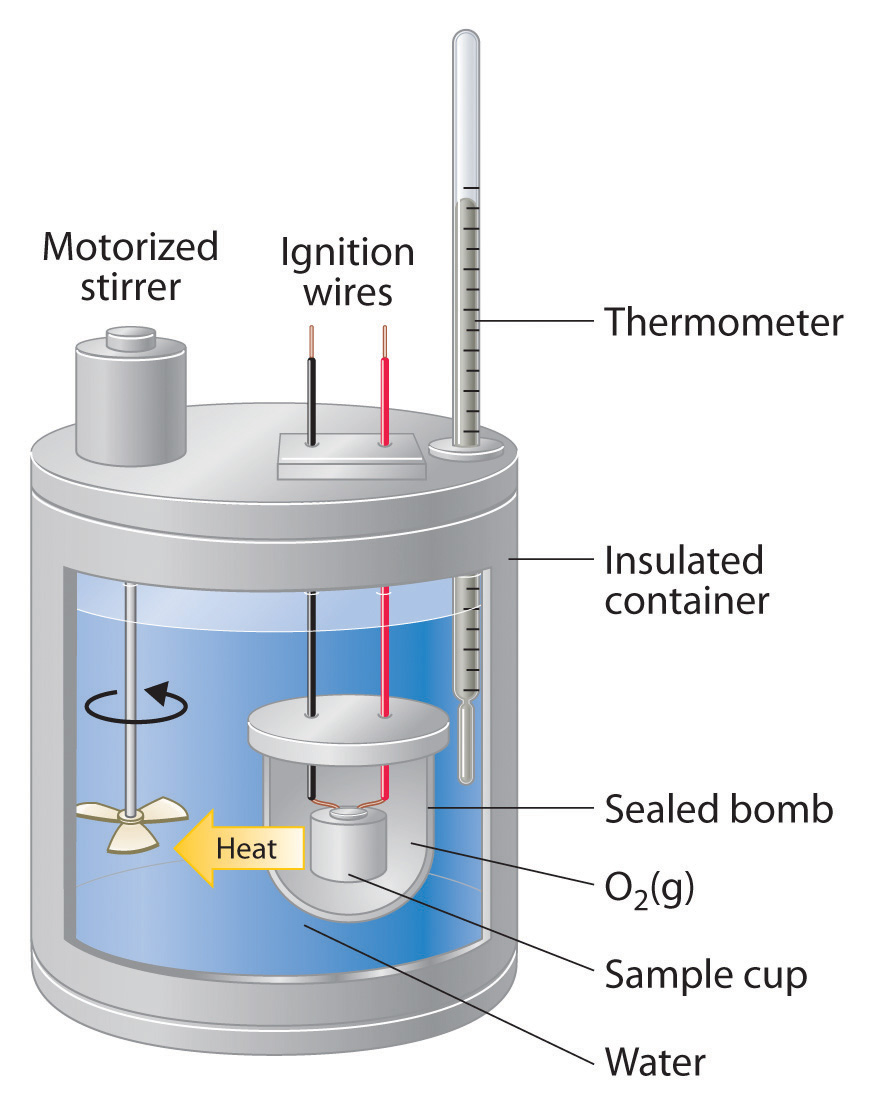
A Calorimeter Is Used To Determine The Quantity Of . One technique we can use to measure the amount of heat involved in a chemical or physical process is. calorimetry is used to measure amounts of heat transferred to or from a substance. determine a calorimeter constant i example #3: A calorimeter is to be calibrated: in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. 72.55 g of water at 71.6 °c added to a calorimeter. To do so, the heat is exchanged with a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Most students likely do not remember using such a.
from chem.libretexts.org
a calorimeter is a device used to measure the quantity of heat transferred to or from an object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. One technique we can use to measure the amount of heat involved in a chemical or physical process is. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is to be calibrated: Most students likely do not remember using such a. 72.55 g of water at 71.6 °c added to a calorimeter. determine a calorimeter constant i example #3: a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
5.3 Calorimetry Chemistry LibreTexts
A Calorimeter Is Used To Determine The Quantity Of a calorimeter is a device used to measure the quantity of heat transferred to or from an object. One technique we can use to measure the amount of heat involved in a chemical or physical process is. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. A calorimeter is to be calibrated: Most students likely do not remember using such a. 72.55 g of water at 71.6 °c added to a calorimeter. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. To do so, the heat is exchanged with a. determine a calorimeter constant i example #3: a calorimeter is a device used to measure the quantity of heat transferred to or from an object.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 A Calorimeter Is Used To Determine The Quantity Of a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a. One technique we can use to measure the amount of heat involved in a chemical or physical process is. calorimetry is used to measure amounts of heat transferred to or. A Calorimeter Is Used To Determine The Quantity Of.
From pathwaystochemistry.com
Calorimetry Pathways to Chemistry A Calorimeter Is Used To Determine The Quantity Of 72.55 g of water at 71.6 °c added to a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Most students likely do not remember using such a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. To do so,. A Calorimeter Is Used To Determine The Quantity Of.
From frankmccomb.info
Which Statement Describes How a Basic Coffee Cup Calorimeter Works? A Calorimeter Is Used To Determine The Quantity Of a calorimeter is a device used to measure the quantity of heat transferred to or from an object. A calorimeter is to be calibrated: Most students likely do not remember using such a. To do so, the heat is exchanged with a. One technique we can use to measure the amount of heat involved in a chemical or physical. A Calorimeter Is Used To Determine The Quantity Of.
From revisionug.com
What is Calorimetry? A Calorimeter Is Used To Determine The Quantity Of calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. A calorimeter is to be calibrated: One technique we can use to measure the amount of heat involved in a chemical or physical process is. a calorimeter is a device used to measure the amount of heat involved in a chemical or. A Calorimeter Is Used To Determine The Quantity Of.
From exoarnnpu.blob.core.windows.net
Calorimeter Description And Function at Kyla Ochs blog A Calorimeter Is Used To Determine The Quantity Of a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is used to measure. A Calorimeter Is Used To Determine The Quantity Of.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec A Calorimeter Is Used To Determine The Quantity Of One technique we can use to measure the amount of heat involved in a chemical or physical process is. determine a calorimeter constant i example #3: Most students likely do not remember using such a. To do so, the heat is exchanged with a. 72.55 g of water at 71.6 °c added to a calorimeter. calorimetry is used. A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube A Calorimeter Is Used To Determine The Quantity Of One technique we can use to measure the amount of heat involved in a chemical or physical process is. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. determine a calorimeter constant i example #3: calorimetry is used to measure amounts of heat transferred to or from a substance. To. A Calorimeter Is Used To Determine The Quantity Of.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry A Calorimeter Is Used To Determine The Quantity Of 72.55 g of water at 71.6 °c added to a calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. A calorimeter is to be calibrated: calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device used to measure. A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube A Calorimeter Is Used To Determine The Quantity Of One technique we can use to measure the amount of heat involved in a chemical or physical process is. A calorimeter is to be calibrated: a calorimeter is a device used to measure the quantity of heat transferred to or from an object. 72.55 g of water at 71.6 °c added to a calorimeter. determine a calorimeter constant. A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle A Calorimeter Is Used To Determine The Quantity Of Most students likely do not remember using such a. 72.55 g of water at 71.6 °c added to a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is. . A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube A Calorimeter Is Used To Determine The Quantity Of a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. calorimetry is used to measure amounts of heat transferred to or from a substance. Most students likely do not remember. A Calorimeter Is Used To Determine The Quantity Of.
From www.slideserve.com
PPT A calorimeter is used to measure the amount of heat absorbed or A Calorimeter Is Used To Determine The Quantity Of a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. determine a calorimeter constant i example #3: calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the quantity of heat transferred to or from. A Calorimeter Is Used To Determine The Quantity Of.
From www.bartleby.com
Answered A calorimeter is used to measure the… bartleby A Calorimeter Is Used To Determine The Quantity Of determine a calorimeter constant i example #3: in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Most students likely do not remember using such a. One technique we can use to measure the amount of heat involved in a chemical or physical process is. A calorimeter is to be calibrated: . A Calorimeter Is Used To Determine The Quantity Of.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter A Calorimeter Is Used To Determine The Quantity Of To do so, the heat is exchanged with a. determine a calorimeter constant i example #3: a calorimeter is a device used to measure the quantity of heat transferred to or from an object. Most students likely do not remember using such a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure'). A Calorimeter Is Used To Determine The Quantity Of.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog A Calorimeter Is Used To Determine The Quantity Of A calorimeter is to be calibrated: determine a calorimeter constant i example #3: 72.55 g of water at 71.6 °c added to a calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is used to measure amounts of heat transferred to or from a substance. Most students likely. A Calorimeter Is Used To Determine The Quantity Of.
From users.highland.edu
Calorimetry A Calorimeter Is Used To Determine The Quantity Of calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. 72.55 g of water at 71.6 °c added to. A Calorimeter Is Used To Determine The Quantity Of.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson A Calorimeter Is Used To Determine The Quantity Of To do so, the heat is exchanged with a. Most students likely do not remember using such a. calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is. A calorimeter is to be calibrated: determine. A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
Principle of Calorimetry YouTube A Calorimeter Is Used To Determine The Quantity Of calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is used to measure amounts of heat transferred to or from a substance. 72.55 g of water at 71.6 °c added to a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical. A Calorimeter Is Used To Determine The Quantity Of.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica A Calorimeter Is Used To Determine The Quantity Of calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. determine a calorimeter constant i example #3: a calorimeter is a device used to measure the quantity of heat transferred to or from an object. Most students likely do not remember using such a. To do so, the heat is exchanged. A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube A Calorimeter Is Used To Determine The Quantity Of To do so, the heat is exchanged with a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. A calorimeter is to be calibrated: 72.55 g of water at 71.6 °c added to a calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the.. A Calorimeter Is Used To Determine The Quantity Of.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts A Calorimeter Is Used To Determine The Quantity Of calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is to be calibrated:. A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube A Calorimeter Is Used To Determine The Quantity Of One technique we can use to measure the amount of heat involved in a chemical or physical process is. 72.55 g of water at 71.6 °c added to a calorimeter. determine a calorimeter constant i example #3: To do so, the heat is exchanged with a. a calorimeter is a device used to measure the quantity of heat. A Calorimeter Is Used To Determine The Quantity Of.
From chemistrytalk.org
Calorimetry ChemTalk A Calorimeter Is Used To Determine The Quantity Of determine a calorimeter constant i example #3: a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is to be calibrated: calorimetry is used to measure amounts of heat transferred to or from a substance. Most students likely do not remember using such a. a. A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube A Calorimeter Is Used To Determine The Quantity Of One technique we can use to measure the amount of heat involved in a chemical or physical process is. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. To do. A Calorimeter Is Used To Determine The Quantity Of.
From www.animalia-life.club
Calorimeter Diagram A Calorimeter Is Used To Determine The Quantity Of A calorimeter is to be calibrated: 72.55 g of water at 71.6 °c added to a calorimeter. To do so, the heat is exchanged with a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a. A Calorimeter Is Used To Determine The Quantity Of.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter A Calorimeter Is Used To Determine The Quantity Of A calorimeter is to be calibrated: Most students likely do not remember using such a. To do so, the heat is exchanged with a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. 72.55 g of water at 71.6 °c added to a calorimeter. calorimetry is used to measure amounts of. A Calorimeter Is Used To Determine The Quantity Of.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep A Calorimeter Is Used To Determine The Quantity Of a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is to be calibrated: in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. To do so, the heat is exchanged with a. One technique we can use to measure the. A Calorimeter Is Used To Determine The Quantity Of.
From www.thoughtco.com
Calorimeter Definition in Chemistry A Calorimeter Is Used To Determine The Quantity Of One technique we can use to measure the amount of heat involved in a chemical or physical process is. determine a calorimeter constant i example #3: To do so, the heat is exchanged with a. A calorimeter is to be calibrated: in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. . A Calorimeter Is Used To Determine The Quantity Of.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts A Calorimeter Is Used To Determine The Quantity Of To do so, the heat is exchanged with a. determine a calorimeter constant i example #3: A calorimeter is to be calibrated: in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter. A Calorimeter Is Used To Determine The Quantity Of.
From www.youtube.com
How to find calorimeter constant YouTube A Calorimeter Is Used To Determine The Quantity Of A calorimeter is to be calibrated: calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Most students likely do not remember using such a. 72.55 g of water at 71.6 °c added to a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical. A Calorimeter Is Used To Determine The Quantity Of.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses A Calorimeter Is Used To Determine The Quantity Of in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. One technique we can use to measure the amount of heat involved in a chemical or physical process is. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a.. A Calorimeter Is Used To Determine The Quantity Of.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog A Calorimeter Is Used To Determine The Quantity Of 72.55 g of water at 71.6 °c added to a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. determine a calorimeter constant i example #3: One technique we can. A Calorimeter Is Used To Determine The Quantity Of.
From scienceinfo.com
Calorimeter Definition, Types and Uses A Calorimeter Is Used To Determine The Quantity Of a calorimeter is a device used to measure the quantity of heat transferred to or from an object. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. determine a calorimeter constant i example #3: 72.55 g of water at 71.6 °c added to a calorimeter. calorimeter, device for measuring. A Calorimeter Is Used To Determine The Quantity Of.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors A Calorimeter Is Used To Determine The Quantity Of calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Most students likely do not remember using such a. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat'. A Calorimeter Is Used To Determine The Quantity Of.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors A Calorimeter Is Used To Determine The Quantity Of in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. To do so, the heat is exchanged with a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. One technique we can use to measure the amount of heat involved in a chemical or physical. A Calorimeter Is Used To Determine The Quantity Of.