Indicator For Oxalic Acid And Sodium Hydroxide . the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. reagents and chemicals for standardization of naoh with oxalic acid. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. for the titration between oxalic acid and sodium hydroxide, which indicator is used? Phenolphthalein is chosen as an. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. The curve is for the reaction.
from www.chegg.com
reagents and chemicals for standardization of naoh with oxalic acid. The curve is for the reaction. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. for the titration between oxalic acid and sodium hydroxide, which indicator is used? the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. Phenolphthalein is chosen as an. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two.
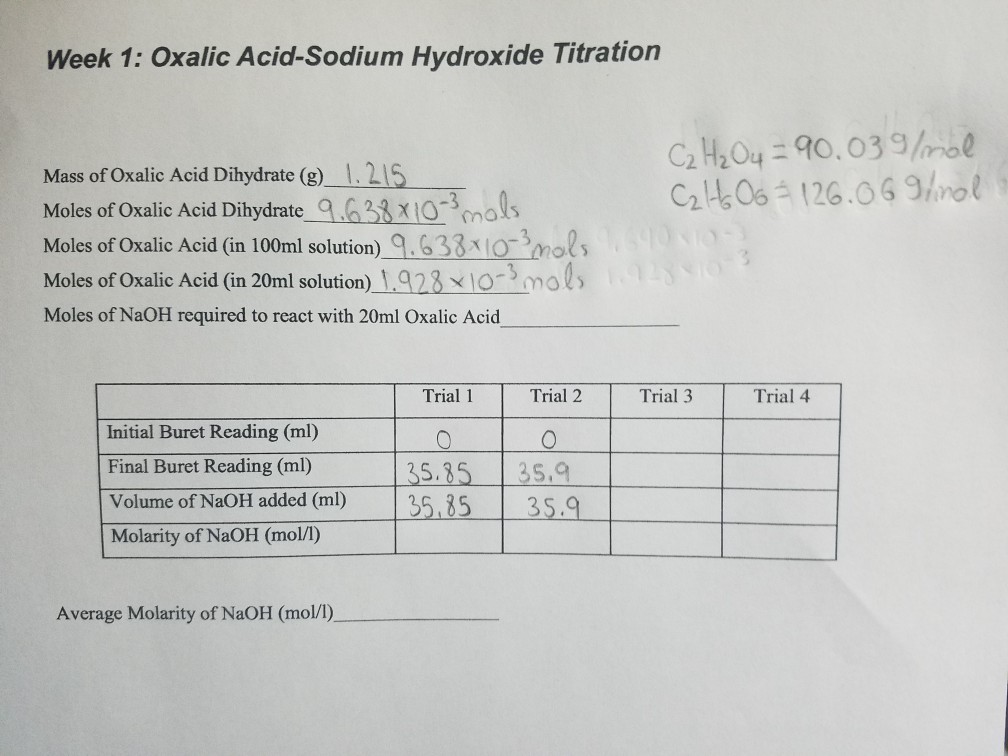
Solved Week 1 Oxalic AcidSodium Hydroxide Titration Mass
Indicator For Oxalic Acid And Sodium Hydroxide this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. reagents and chemicals for standardization of naoh with oxalic acid. for the titration between oxalic acid and sodium hydroxide, which indicator is used? the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. Phenolphthalein is chosen as an. The curve is for the reaction. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions.
From www.youtube.com
Estimation of Oxalic Acid V/S Sodium Hydroxide YouTube Indicator For Oxalic Acid And Sodium Hydroxide Phenolphthalein is chosen as an. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. The curve is for the reaction. if you run sodium hydroxide solution into. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.scribd.com
Chem Lab Report Oxalic Acid Titration Sodium Hydroxide Indicator For Oxalic Acid And Sodium Hydroxide if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. Phenolphthalein is chosen as an. reagents and chemicals for standardization of naoh with oxalic acid. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. this experiment involves titrating a weak acid, oxalic acid, with. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.tutormyself.com
233 Archives TutorMyself Chemistry Indicator For Oxalic Acid And Sodium Hydroxide the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. The curve is for the reaction. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.ase.org.uk
Science Note Titration curve of ethanedioic acid (oxalic acid) with Indicator For Oxalic Acid And Sodium Hydroxide if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. Phenolphthalein is chosen as an. The curve is for the reaction. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. for the titration between oxalic. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
Sodium Hydroxide Standardization with Oxalic acid YouTube Indicator For Oxalic Acid And Sodium Hydroxide reagents and chemicals for standardization of naoh with oxalic acid. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. The curve is for the reaction. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
Titration curve for sodium hydroxide and oxalic acid YouTube Indicator For Oxalic Acid And Sodium Hydroxide for the titration between oxalic acid and sodium hydroxide, which indicator is used? if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. reagents and chemicals for standardization of naoh with oxalic acid. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. this. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.numerade.com
SOLVED The graph below shows the titration of oxalic acid, C2H2O4 Indicator For Oxalic Acid And Sodium Hydroxide if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. for the titration between oxalic acid and sodium hydroxide, which indicator is used? Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. reagents and chemicals for standardization of naoh with oxalic acid. this. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.studypool.com
SOLUTION Titration of oxalic acid against sodium hydroxide Studypool Indicator For Oxalic Acid And Sodium Hydroxide The curve is for the reaction. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. . Indicator For Oxalic Acid And Sodium Hydroxide.
From www.toppr.com
What is the indicator used for the titration between oxalic acid and Indicator For Oxalic Acid And Sodium Hydroxide the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. Phenolphthalein is chosen as an. for the titration between oxalic acid and sodium hydroxide, which indicator is used?. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.scribd.com
Titration of Sodium Hydroxide by Using Oxalic Acid 4 PDF Titration Indicator For Oxalic Acid And Sodium Hydroxide the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. for the titration between oxalic acid and sodium hydroxide, which indicator is used? the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. The curve is for the reaction. Phenolphthalein is. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.doubtnut.com
[Tamil] For the titration between oxalic acid and sodium hydroxide, th Indicator For Oxalic Acid And Sodium Hydroxide this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. for the titration between oxalic acid and sodium hydroxide, which indicator is used? the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. Phenolphthalein is chosen as an. if. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.studocu.com
Determine the strength of the given sodium hydroxide solution by Indicator For Oxalic Acid And Sodium Hydroxide reagents and chemicals for standardization of naoh with oxalic acid. The curve is for the reaction. Phenolphthalein is chosen as an. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two.. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.brightpharma.in
Discuss the preparation and standardization of Oxalic acid or Sodium Indicator For Oxalic Acid And Sodium Hydroxide Phenolphthalein is chosen as an. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. reagents and chemicals for standardization of naoh with oxalic acid. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. . Indicator For Oxalic Acid And Sodium Hydroxide.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Indicator For Oxalic Acid And Sodium Hydroxide if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. reagents and chemicals for standardization of naoh with oxalic acid. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. for the titration between oxalic acid and. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.studypool.com
SOLUTION Titration of oxalic acid against sodium hydroxide Studypool Indicator For Oxalic Acid And Sodium Hydroxide Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. Phenolphthalein is chosen as an. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. . Indicator For Oxalic Acid And Sodium Hydroxide.
From www.studypool.com
SOLUTION Titration of oxalic acid against sodium hydroxide Studypool Indicator For Oxalic Acid And Sodium Hydroxide the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. Phenolphthalein is chosen as an. for the titration between oxalic acid and sodium hydroxide, which indicator is used? Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. the experiment revolves around the titration of oxalic acid (a. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.scribd.com
BCB 103L Expt 6 Titration of Sodium Hydroxide With Oxalic Acid Using Indicator For Oxalic Acid And Sodium Hydroxide for the titration between oxalic acid and sodium hydroxide, which indicator is used? Phenolphthalein is chosen as an. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
Titration of Sodium Hydroxide Solution by Oxalic Acid Solution Indicator For Oxalic Acid And Sodium Hydroxide the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. Phenolphthalein is chosen as an. The curve is for the reaction. if you run sodium hydroxide solution into ethanedioic acid solution,. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
Which of following indicators is used in the titration of oxalic acid Indicator For Oxalic Acid And Sodium Hydroxide this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.chegg.com
Solved (II) Analysis of a mixture of oxalic acid and sodium Indicator For Oxalic Acid And Sodium Hydroxide reagents and chemicals for standardization of naoh with oxalic acid. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. for the titration between oxalic acid and sodium hydroxide, which indicator is used? The curve is for. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
PREPARATION AND STANDARDIZATION OF VARIOUS MOLAR AND NORMAL SOLUTIONS Indicator For Oxalic Acid And Sodium Hydroxide Phenolphthalein is chosen as an. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. The curve is for the reaction. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. if you run sodium hydroxide solution into. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.numerade.com
SOLVED write balanced chemical equation between oxalic acid and sodium Indicator For Oxalic Acid And Sodium Hydroxide this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. reagents and chemicals for standardization of naoh with oxalic acid. the mass of oxalic acid dihydrate, the volume of. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.brightpharma.in
Discuss the preparation and standardization of Oxalic acid or Sodium Indicator For Oxalic Acid And Sodium Hydroxide The curve is for the reaction. for the titration between oxalic acid and sodium hydroxide, which indicator is used? this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. Phenolphthalein is chosen as an. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
FSc class chemistry practical, standardization of Sodium Hydroxide with Indicator For Oxalic Acid And Sodium Hydroxide this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. Phenolphthalein is chosen as an. for the titration between oxalic acid and sodium hydroxide, which indicator is used? reagents and chemicals for standardization of naoh with oxalic acid. the mass of oxalic acid dihydrate, the volume. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.toppr.com
What is the indicator used for the titration between oxalic acid and Indicator For Oxalic Acid And Sodium Hydroxide reagents and chemicals for standardization of naoh with oxalic acid. The curve is for the reaction. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.numerade.com
SOLVED Consider the titration curve below for the titration of oxalic Indicator For Oxalic Acid And Sodium Hydroxide Phenolphthalein is chosen as an. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. for the titration between oxalic acid and sodium hydroxide, which indicator is used? reagents and. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.studypool.com
SOLUTION Estimation of oxalic acid and sodium oxalate pptx Studypool Indicator For Oxalic Acid And Sodium Hydroxide this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. The curve is for the reaction. Phenolphthalein is chosen as an. for the titration between oxalic acid and sodium hydroxide, which indicator is used? the experiment revolves around the titration of oxalic acid (a weak acid) against. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
Estimation of Sodium hydroxide V/S Oxalic acid YouTube Indicator For Oxalic Acid And Sodium Hydroxide reagents and chemicals for standardization of naoh with oxalic acid. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. if you run sodium hydroxide solution into. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
Standardization of NaOH Vs Oxalic acid Lab Activity Oxalic Acid as a Indicator For Oxalic Acid And Sodium Hydroxide Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. Phenolphthalein is chosen as an. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. reagents and chemicals for standardization of naoh with oxalic acid. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide,. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
TITRATION OXALIC ACID VS SODIUM HYDROXIDE SOLUTION OXALIC ACID VS Indicator For Oxalic Acid And Sodium Hydroxide reagents and chemicals for standardization of naoh with oxalic acid. for the titration between oxalic acid and sodium hydroxide, which indicator is used? the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.studypool.com
SOLUTION Titration of oxalic acid against sodium hydroxide Studypool Indicator For Oxalic Acid And Sodium Hydroxide reagents and chemicals for standardization of naoh with oxalic acid. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these reactions. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide,. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
CLASS XI TOPIC TITRATION OXALIC ACID AND SODIUM HYDROXIDE Indicator For Oxalic Acid And Sodium Hydroxide Phenolphthalein is chosen as an. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. The curve is for the reaction. Sodium hydroxide naoh and oxalic acid (cooh) 2.2h 2 o. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong.. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.youtube.com
Preparation and Standardization of Sodium Hydroxide with Oxalic Acid I Indicator For Oxalic Acid And Sodium Hydroxide The curve is for the reaction. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. for the titration between oxalic acid and sodium hydroxide, which indicator is used? if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end points for both of these. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.chegg.com
Solved Week 1 Oxalic AcidSodium Hydroxide Titration Mass Indicator For Oxalic Acid And Sodium Hydroxide the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. the mass of oxalic acid dihydrate, the volume of sodium hydroxide used, and the stoichiometric relationship of these two. Phenolphthalein is chosen as an. if you run sodium hydroxide solution into ethanedioic acid solution, the ph curve shows the end. Indicator For Oxalic Acid And Sodium Hydroxide.
From www.numerade.com
Volumetric Analysis Standardization of Sodium Hydroxide Solution Data Indicator For Oxalic Acid And Sodium Hydroxide The curve is for the reaction. reagents and chemicals for standardization of naoh with oxalic acid. this experiment involves titrating a weak acid, oxalic acid, with a strong base, sodium hydroxide, using phenolphthalein as an indicator. the experiment revolves around the titration of oxalic acid (a weak acid) against sodium hydroxide (a strong. Sodium hydroxide naoh and. Indicator For Oxalic Acid And Sodium Hydroxide.