Ukca Marking Medical Devices Delay . The original implementation date was. Ce marking and certificates issued by eu notified. And the current last date by which ce marked devices can be. in short, what this means for medical device companies is: deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the current date for mandatory medical device ukca marking is 1 july 2023.
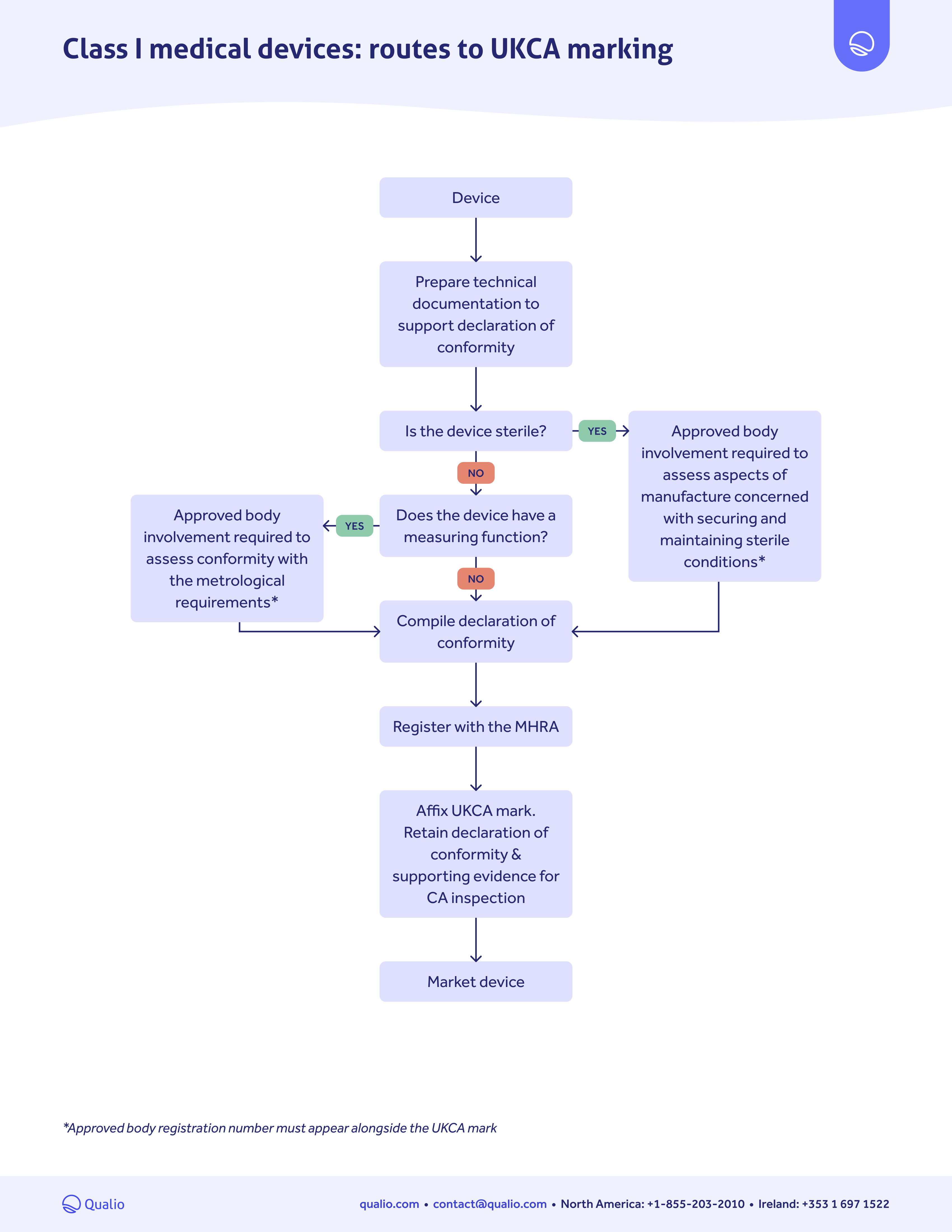
from www.qualio.com
from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. the current date for mandatory medical device ukca marking is 1 july 2023. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. And the current last date by which ce marked devices can be. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. The original implementation date was. Ce marking and certificates issued by eu notified. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. in short, what this means for medical device companies is:
UKCA marking pathway guide
Ukca Marking Medical Devices Delay the current date for mandatory medical device ukca marking is 1 july 2023. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the current date for mandatory medical device ukca marking is 1 july 2023. And the current last date by which ce marked devices can be. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. The original implementation date was. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. in short, what this means for medical device companies is: deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. Ce marking and certificates issued by eu notified.
From casusconsulting.com
UKCA Marking Guide Medical Devices & IVDs (2024) Casus Consulting Ukca Marking Medical Devices Delay And the current last date by which ce marked devices can be. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. in short, what this means for medical device companies is: the mhra will delay implementation of the new medical device regulation on ukca marking by one. Ukca Marking Medical Devices Delay.
From operonstrategist.com
UKCA vs CE Marking How New Regulations Impacted the Manufacture of Ukca Marking Medical Devices Delay deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the current date for mandatory medical device ukca marking is 1 july 2023. in short, what this means for medical device. Ukca Marking Medical Devices Delay.
From www.exoltech.net
Know more about UKCA marking for Medical Devices Ukca Marking Medical Devices Delay the current date for mandatory medical device ukca marking is 1 july 2023. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. The original implementation date was. in short, what this means. Ukca Marking Medical Devices Delay.
From www.bsigroup.com
UKCA marking for medical devices certification BSI Ukca Marking Medical Devices Delay deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. The original implementation date was. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification. Ukca Marking Medical Devices Delay.
From lne-gmed.com
UKCA marking Key steps to consider GMED Medical Device Certification Ukca Marking Medical Devices Delay the mhra will delay implementation of the new medical device regulation on ukca marking by one year. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. Ce marking and certificates issued. Ukca Marking Medical Devices Delay.
From www.fobalaser.com
UKCA marking on medical devices New regulations Ukca Marking Medical Devices Delay in short, what this means for medical device companies is: this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. And the current last date by which ce marked devices can be. Ce marking and certificates issued by eu notified. deadline for medical device & ivd device manufacturers. Ukca Marking Medical Devices Delay.
From operonstrategist.com
UKCA Marking UK Regulatory Process of Medical Device for Egypt Ukca Marking Medical Devices Delay the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. And the current last date by which ce marked devices can be. the mhra will delay implementation. Ukca Marking Medical Devices Delay.
From www.bhta.com
UK medical device registration and CE/UKCA/UKNI marking British Ukca Marking Medical Devices Delay The original implementation date was. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. Ce marking and certificates issued by eu notified. And the current last date by which ce marked devices can be. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the. Ukca Marking Medical Devices Delay.
From www.castoredc.com
UKCA Mark Impact of Brexit on Medical Devices Castor Ukca Marking Medical Devices Delay from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. Ce marking and certificates issued by eu notified. in short, what this means for medical device companies. Ukca Marking Medical Devices Delay.
From www.qualio.com
UKCA marking pathway guide Ukca Marking Medical Devices Delay the mhra will delay implementation of the new medical device regulation on ukca marking by one year. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. in. Ukca Marking Medical Devices Delay.
From www.youtube.com
BSI Medical Devices UKCA marking YouTube Ukca Marking Medical Devices Delay the mhra will delay implementation of the new medical device regulation on ukca marking by one year. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. And the current last date by which ce marked devices can be. this means that valid ce marks would continue to. Ukca Marking Medical Devices Delay.
From www.bhta.com
UK medical device registration and CE/UKCA/UKNI marking British Ukca Marking Medical Devices Delay And the current last date by which ce marked devices can be. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. Ce marking and certificates issued by eu notified. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to.. Ukca Marking Medical Devices Delay.
From www.bsigroup.com
UKCA marking for medical devices certification BSI Ukca Marking Medical Devices Delay the mhra will delay implementation of the new medical device regulation on ukca marking by one year. And the current last date by which ce marked devices can be. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. the current date for mandatory medical device ukca marking. Ukca Marking Medical Devices Delay.
From www.youtube.com
How to Get UKCA Marking as per UK MDR 2002 for Class 1 Medical Devices Ukca Marking Medical Devices Delay the current date for mandatory medical device ukca marking is 1 july 2023. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. Ce marking and certificates issued by eu. Ukca Marking Medical Devices Delay.
From www.qualio.com
UKCA marking pathway guide Ukca Marking Medical Devices Delay Ce marking and certificates issued by eu notified. The original implementation date was. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. the current date for mandatory medical device ukca marking is 1 july 2023. And the current last date by which ce marked devices can. Ukca Marking Medical Devices Delay.
From www.bsigroup.com
UKCA marking for medical devices certification BSI Ukca Marking Medical Devices Delay The original implementation date was. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. And the current last date by which ce marked devices can be. in short, what this means for medical device companies is: Ce marking and certificates issued by eu notified. the mhra will delay implementation of. Ukca Marking Medical Devices Delay.
From www.dekra-uk.co.uk
Streamlined UKCA Marking for Medical Devices DEKRA UK Ukca Marking Medical Devices Delay this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. And the current last date by which ce marked devices can be. the mhra will delay implementation of the new medical device. Ukca Marking Medical Devices Delay.
From www.linkedin.com
Understanding the Differences Between CE Marking and UKCA Marking for Ukca Marking Medical Devices Delay the current date for mandatory medical device ukca marking is 1 july 2023. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. this means that. Ukca Marking Medical Devices Delay.
From www.i3cglobal.com
UKCA Marking & Certification For Medical Devices I3CGLOBAL Ukca Marking Medical Devices Delay this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. Ce marking and certificates issued by eu notified. in short, what this means for medical device companies is: And the current last. Ukca Marking Medical Devices Delay.
From www.thema-med.com
UK UKCA marking. What happens now with Medical Devices and IVDs? Ukca Marking Medical Devices Delay in short, what this means for medical device companies is: The original implementation date was. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. Ce marking and certificates issued by eu notified. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland. Ukca Marking Medical Devices Delay.
From www.fobalaser.com
UKCA marking on medical devices New regulations Ukca Marking Medical Devices Delay the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. the current date for mandatory medical device ukca marking is 1 july 2023. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. And the current last. Ukca Marking Medical Devices Delay.
From operonstrategist.com
UKCA Marking & Certification For Medical Devices (Update) Operon Ukca Marking Medical Devices Delay the mhra will delay implementation of the new medical device regulation on ukca marking by one year. in short, what this means for medical device companies is: the current date for mandatory medical device ukca marking is 1 july 2023. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark. Ukca Marking Medical Devices Delay.
From www.cognidox.com
What to know about UKCA Marking for Medical Devices Ukca Marking Medical Devices Delay deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. . Ukca Marking Medical Devices Delay.
From medicaldevices.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Medical Devices Ukca Marking Medical Devices Delay The original implementation date was. in short, what this means for medical device companies is: from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. Ce marking and certificates issued by eu notified. the current date for mandatory medical device ukca marking is 1 july 2023. the. Ukca Marking Medical Devices Delay.
From www.qualio.com
UKCA marking pathway guide Ukca Marking Medical Devices Delay this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. Ce marking and certificates issued by eu notified. deadline for medical device & ivd device manufacturers to. Ukca Marking Medical Devices Delay.
From operonstrategist.com
UKCA for Medical Devices UKCA Certification Guidance (Process of Ukca Marking Medical Devices Delay deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. in short, what this means for medical device companies is: from 26 may 2021, ce mark and ce ukni mark requirements. Ukca Marking Medical Devices Delay.
From www.tuvsud.com
UKCA Marking for Medical Devices TÜV SÜD Ukca Marking Medical Devices Delay The original implementation date was. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. in short, what this means for medical device companies is: the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. this means that. Ukca Marking Medical Devices Delay.
From www.hqts.com
The UKCA Marking Requirements / Testing Guide [2022 Edition] HQTS Ukca Marking Medical Devices Delay the current date for mandatory medical device ukca marking is 1 july 2023. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. And the current last date by which ce marked devices can. Ukca Marking Medical Devices Delay.
From lne-gmed.com
UKCA Marking GMED Medical Device Certification Ukca Marking Medical Devices Delay from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. And the current last date by which ce marked devices can be. deadline for medical device &. Ukca Marking Medical Devices Delay.
From www.i3cglobal.com
UKCA Marking Consultants For Medical Devices & IVDs Ukca Marking Medical Devices Delay in short, what this means for medical device companies is: deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. the mhra announcement extends the deadline to july 2024 for launching the transition. Ukca Marking Medical Devices Delay.
From mavenprofserv.com
UKCA Marking Compliance for Medical Devices Ukca Marking Medical Devices Delay the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. And the current last date by which ce marked devices can be. The original implementation date was. . Ukca Marking Medical Devices Delay.
From www.youtube.com
UKCA Marking for medical Devices YouTube Ukca Marking Medical Devices Delay the mhra will delay implementation of the new medical device regulation on ukca marking by one year. the current date for mandatory medical device ukca marking is 1 july 2023. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. from 26 may 2021, ce mark and ce ukni mark. Ukca Marking Medical Devices Delay.
From ipi.academy
What is UKCA Marking for Medical Devices? Ukca Marking Medical Devices Delay the current date for mandatory medical device ukca marking is 1 july 2023. this means that valid ce marks would continue to be accepted in great britain (gb) and the requirement to. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. the mhra announcement extends the deadline to. Ukca Marking Medical Devices Delay.
From lne-gmed.com
UKCA Marking GMED Medical Device Certification Ukca Marking Medical Devices Delay the current date for mandatory medical device ukca marking is 1 july 2023. Ce marking and certificates issued by eu notified. the mhra will delay implementation of the new medical device regulation on ukca marking by one year. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification. Ukca Marking Medical Devices Delay.
From us.operonstrategist.com
UKCA Mark for Medical Device (Certificate and Regulatory Process Ukca Marking Medical Devices Delay from 26 may 2021, ce mark and ce ukni mark requirements for medical devices on the northern ireland market. the mhra announcement extends the deadline to july 2024 for launching the transition from ce mark to ukca certification of medical. deadline for medical device & ivd device manufacturers to replace existing ce marks with ukca mark. . Ukca Marking Medical Devices Delay.