Standard Reduction Potential Conditions . Use standard reduction potentials to determine the. Use standard reduction potentials to determine the better oxidizing or. What are the differences between the standard reduction potential and. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. By the end of this module, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What does the standard reduction potential measure? The standard reduction potential is the reduction potential of a molecule under specific, standard conditions.
from www.chegg.com
What are the differences between the standard reduction potential and. Use standard reduction potentials to determine the. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What does the standard reduction potential measure? Use standard reduction potentials to determine the better oxidizing or. By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions.
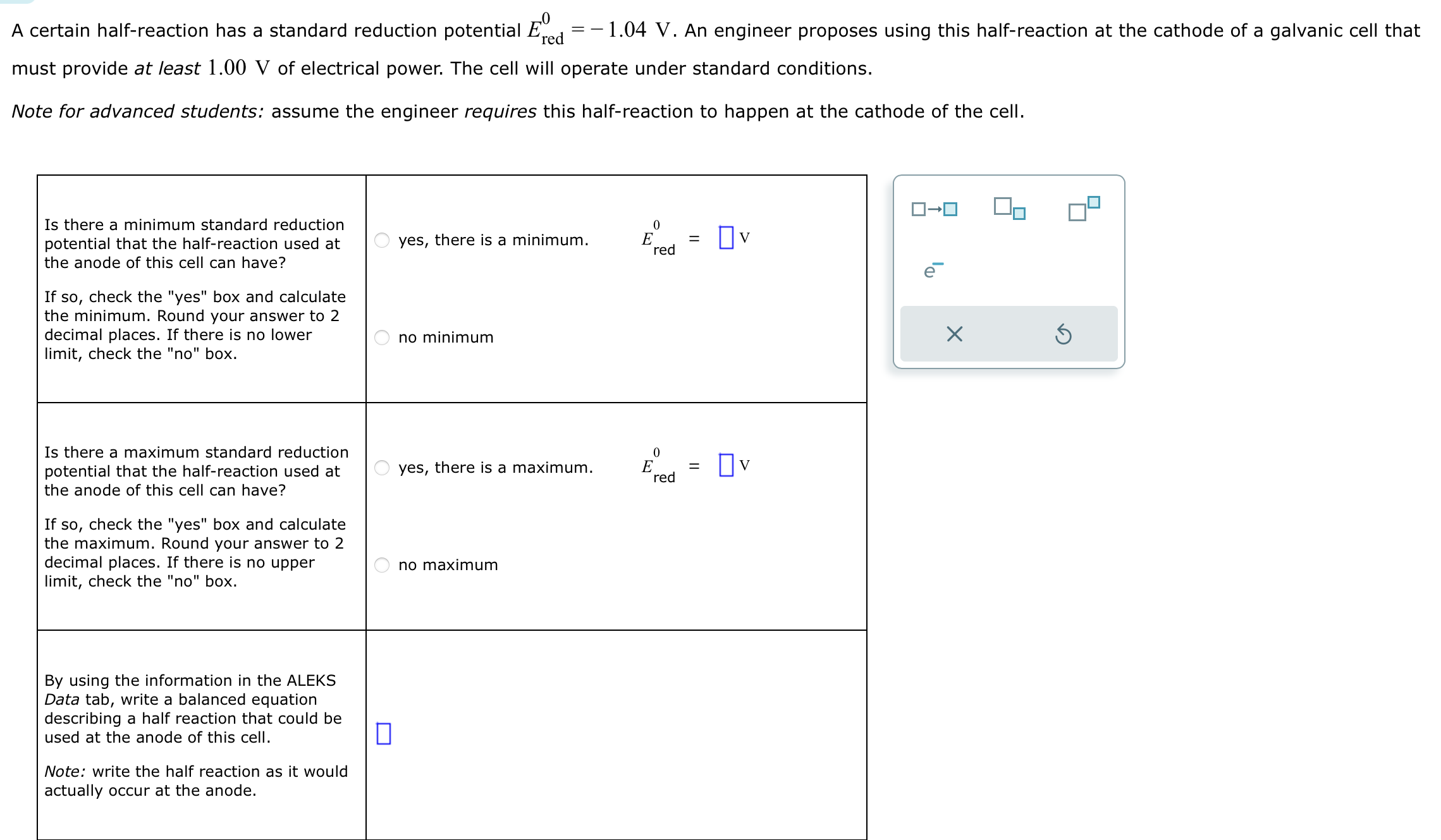
Solved certain halfreaction has a standard reduction
Standard Reduction Potential Conditions The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. By the end of this module, you will be able to: Use standard reduction potentials to determine the. What are the differences between the standard reduction potential and. Use standard reduction potentials to determine the better oxidizing or. What does the standard reduction potential measure? The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Conditions By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a. Standard Reduction Potential Conditions.
From www.chegg.com
Solved 17. Using standard reduction potentials (on the next Standard Reduction Potential Conditions What does the standard reduction potential measure? The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions.. Standard Reduction Potential Conditions.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. Use standard reduction potentials to determine the better oxidizing or. What does the standard reduction potential measure? Use standard reduction potentials to determine the. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID5480021 Standard Reduction Potential Conditions Use standard reduction potentials to determine the better oxidizing or. By the end of this module, you will be able to: Use standard reduction potentials to determine the. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the reduction potential of. Standard Reduction Potential Conditions.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Conditions Use standard reduction potentials to determine the. By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What are the differences between the standard reduction potential and. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. Use standard. Standard Reduction Potential Conditions.
From www.toppr.com
The standard reduction potential at 25^∘C of the reaction 2H2O + 2e^→ H2 + 2OH^ is 0.8277 Standard Reduction Potential Conditions By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What does the standard reduction potential measure? The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known as the standard electrode potential, is. Standard Reduction Potential Conditions.
From www.chegg.com
Solved certain halfreaction has a standard reduction Standard Reduction Potential Conditions What are the differences between the standard reduction potential and. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. Use standard reduction potentials to determine the. By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard. Standard Reduction Potential Conditions.
From www.numerade.com
SOLVEDExample 4 Using standard reduction potentials determine which of the following reactions Standard Reduction Potential Conditions The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What does the standard reduction potential measure? By the end of this module, you will be able to: Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the reduction potential of. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download ID4653489 Standard Reduction Potential Conditions Use standard reduction potentials to determine the. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What does the standard reduction potential measure? Use standard reduction potentials to determine the better. Standard Reduction Potential Conditions.
From solvedlib.com
Using the standard reduction potentials, calculate th… SolvedLib Standard Reduction Potential Conditions Use standard reduction potentials to determine the. What does the standard reduction potential measure? The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What are the differences between the standard reduction potential and. By the end of this module, you will be able to: Use standard. Standard Reduction Potential Conditions.
From www.chegg.com
Solved Refer to the table of standard reduction potentials Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. Use standard reduction potentials to determine the. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. By the end of this module, you will be able to: The standard. Standard Reduction Potential Conditions.
From www.youtube.com
Standard Reduction Potentials of Half Reactions Electrochemistry YouTube Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What does the standard reduction potential measure? The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. By the end of this module, you will be able to: What are the differences between the standard reduction potential and. The standard. Standard Reduction Potential Conditions.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Conditions What are the differences between the standard reduction potential and. By the end of this module, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the. Use standard reduction potentials to determine the better oxidizing. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT STANDARD REDUCTION POTENTIAL PowerPoint Presentation, free download ID3000316 Standard Reduction Potential Conditions Use standard reduction potentials to determine the. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire.. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT Intersection 13 PowerPoint Presentation, free download ID567059 Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. Use standard reduction potentials to determine the better oxidizing or. What are the differences between the standard reduction potential. Standard Reduction Potential Conditions.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Conditions What does the standard reduction potential measure? By the end of this module, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What are the differences between the standard reduction potential and. The standard reduction potential is the reduction potential of. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT The oxidation states of vanadium PowerPoint Presentation ID467926 Standard Reduction Potential Conditions Use standard reduction potentials to determine the. What does the standard reduction potential measure? What are the differences between the standard reduction potential and. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known. Standard Reduction Potential Conditions.
From www.chegg.com
Solved 1 homework point(s) Relative Reduction Potential Standard Reduction Potential Conditions What does the standard reduction potential measure? What are the differences between the standard reduction potential and. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the reduction potential of a. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation, free download ID1952136 Standard Reduction Potential Conditions What does the standard reduction potential measure? Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the. The standard reduction potential is the reduction potential of a molecule under specific,. Standard Reduction Potential Conditions.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Conditions The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the. By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What does the standard. Standard Reduction Potential Conditions.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Conditions What does the standard reduction potential measure? Use standard reduction potentials to determine the. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What are the differences between the standard reduction potential and. By the end of this module,. Standard Reduction Potential Conditions.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Conditions Use standard reduction potentials to determine the. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What does the standard reduction potential measure? The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species. Standard Reduction Potential Conditions.
From www.vrogue.co
Standard Reduction Potential Table Reduction Potentia vrogue.co Standard Reduction Potential Conditions Use standard reduction potentials to determine the. What are the differences between the standard reduction potential and. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. By the end of this module, you will be able to: What does. Standard Reduction Potential Conditions.
From www.numerade.com
Selective Oxidation The standard reduction potential for the halfreaction Sn4+ 2e Sn2+ is +0.15 Standard Reduction Potential Conditions The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What are the differences between the standard reduction potential and. Use. Standard Reduction Potential Conditions.
From www.researchgate.net
Values of the standard potentials of cerium redox systems in anodic... Download Table Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. Use standard reduction potentials to determine the. By the end of this module, you will be able to: What does the standard reduction potential measure? The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also. Standard Reduction Potential Conditions.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Lab Manual Standard Reduction Potential Conditions What does the standard reduction potential measure? Use standard reduction potentials to determine the. What are the differences between the standard reduction potential and. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. By the end of this module, you will be able to:. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT Chemistry 1011 PowerPoint Presentation, free download ID5404690 Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What are the differences between the standard reduction potential and. Use standard reduction potentials to determine the better oxidizing or. By the. Standard Reduction Potential Conditions.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Standard Reduction Potential Conditions What are the differences between the standard reduction potential and. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. By the end of this module, you will be able to: What does the standard reduction potential. Standard Reduction Potential Conditions.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What does the standard reduction potential measure? Use standard reduction potentials to determine the. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What are the differences between the standard reduction. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT Product stabilizations in hydrolysis PowerPoint Presentation, free download ID3696784 Standard Reduction Potential Conditions By the end of this module, you will be able to: The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. What are the differences between the standard reduction potential and. The standard reduction potential, also known as the standard. Standard Reduction Potential Conditions.
From www.numerade.com
SOLVED Selective Reduction The standard reduction potential for the halfreaction Sn4+ + 2e Standard Reduction Potential Conditions The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. Use standard reduction potentials to determine the. What are the differences between the standard reduction potential and. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard reduction potential, also known as the standard electrode potential, is a. Standard Reduction Potential Conditions.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4063431 Standard Reduction Potential Conditions By the end of this module, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the better oxidizing or. What are the differences between the standard reduction potential and. What does the standard reduction potential. Standard Reduction Potential Conditions.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential Conditions What are the differences between the standard reduction potential and. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the reduction potential of a molecule under specific, standard conditions. The standard. Standard Reduction Potential Conditions.
From www.toppr.com
The standard reduction potentials for two reactions are given below AgCl (s)+e^→Ag (s) + Cl Standard Reduction Potential Conditions Use standard reduction potentials to determine the. By the end of this module, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the better oxidizing or. What does the standard reduction potential measure? What are. Standard Reduction Potential Conditions.