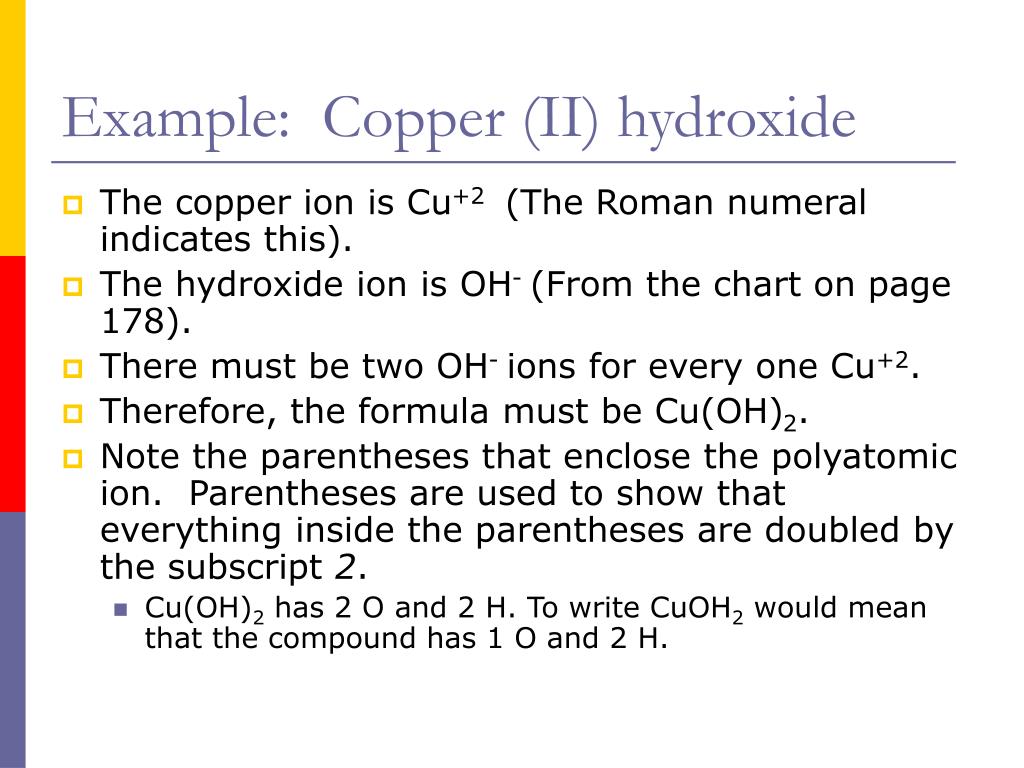
What Happens When Copper Ii Hydroxide Is Heated . Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When it is mixed with copper (ii) carbonate, it is greenish. It reacts with carbon dioxide to make copper (ii) carbonate when in air. Sodium hydroxide precipitates copper (ii) hydroxide: When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. But isn't it a solid by itself too?
from www.slideserve.com
Sodium hydroxide precipitates copper (ii) hydroxide: When it is mixed with copper (ii) carbonate, it is greenish. When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. It reacts with carbon dioxide to make copper (ii) carbonate when in air. But isn't it a solid by itself too? Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated.
PPT Polyatomic Ions and Their Compounds PowerPoint Presentation ID153488
What Happens When Copper Ii Hydroxide Is Heated As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. When it is mixed with copper (ii) carbonate, it is greenish. But isn't it a solid by itself too? Sodium hydroxide precipitates copper (ii) hydroxide: Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. It reacts with carbon dioxide to make copper (ii) carbonate when in air. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide.
From questions-in.kunduz.com
What happens when copper is heated in air... Physical Chemistry What Happens When Copper Ii Hydroxide Is Heated When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. When it is mixed with copper (ii) carbonate, it is greenish. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a. What Happens When Copper Ii Hydroxide Is Heated.
From blog.thepipingmart.com
What Happens When Copper Is Heated In Air? What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When it is mixed with copper (ii) carbonate, it is greenish. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by itself too? When copper(ii) ions react with hydroxide ions in solution, they. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube What Happens When Copper Ii Hydroxide Is Heated As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. It reacts with carbon dioxide to make copper (ii) carbonate when in air. When it is mixed with copper (ii) carbonate, it is greenish. Sodium hydroxide precipitates copper (ii) hydroxide: But isn't it a solid by itself too?. What Happens When Copper Ii Hydroxide Is Heated.
From edata.stfc.ac.uk
Copper (II) hydroxide What Happens When Copper Ii Hydroxide Is Heated But isn't it a solid by itself too? It reacts with carbon dioxide to make copper (ii) carbonate when in air. When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. As. What Happens When Copper Ii Hydroxide Is Heated.
From handwiki.org
ChemistryCopper(II) hydroxide HandWiki What Happens When Copper Ii Hydroxide Is Heated But isn't it a solid by itself too? It reacts with carbon dioxide to make copper (ii) carbonate when in air. Sodium hydroxide precipitates copper (ii) hydroxide: Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When copper(ii) ions react with hydroxide ions in solution, they form the solid,. What Happens When Copper Ii Hydroxide Is Heated.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Science online What Happens When Copper Ii Hydroxide Is Heated Sodium hydroxide precipitates copper (ii) hydroxide: So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. But isn't it a solid by itself too? When it is mixed with copper (ii) carbonate, it is greenish. When copper(ii) ions react. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
of Copper(II) Carbonate Hydroxide Hydrate in LateNiteLabs YouTube What Happens When Copper Ii Hydroxide Is Heated When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Sodium hydroxide precipitates copper (ii) hydroxide: It reacts with carbon dioxide to make copper (ii) carbonate when in air. When it is mixed with copper (ii) carbonate, it is greenish. But. What Happens When Copper Ii Hydroxide Is Heated.
From www.numerade.com
SOLVED When copper (II) oxide is heated in the presence of hydrogen gas, elemental copper and What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide.. What Happens When Copper Ii Hydroxide Is Heated.
From fphoto.com
Fundamental Photographs The Art of Science What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. But isn't it a solid by itself too? When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Sodium hydroxide precipitates. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium hydroxide (NaOH)? Cu(NO3)2 What Happens When Copper Ii Hydroxide Is Heated When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. Sodium hydroxide precipitates copper (ii) hydroxide: So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. It reacts with. What Happens When Copper Ii Hydroxide Is Heated.
From www.sciencephoto.com
Heating copper (II) carbonate Stock Image C010/9635 Science Photo Library What Happens When Copper Ii Hydroxide Is Heated It reacts with carbon dioxide to make copper (ii) carbonate when in air. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When it is mixed with copper (ii) carbonate, it is greenish. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid. What Happens When Copper Ii Hydroxide Is Heated.
From www.sciencephoto.com
Precipitation of copper (II) hydroxide Stock Image A500/0116 Science Photo Library What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. But isn't it a solid by itself too? It reacts with carbon dioxide to make copper (ii) carbonate when in air. When it is mixed with copper (ii) carbonate, it is greenish. So just wondering, copper(ii) hydroxide undergoes thermal decomposition. What Happens When Copper Ii Hydroxide Is Heated.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free download ID2858272 What Happens When Copper Ii Hydroxide Is Heated When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. But isn't it a solid by itself too? It reacts with carbon dioxide to make copper (ii) carbonate when in air. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. As. What Happens When Copper Ii Hydroxide Is Heated.
From www.numerade.com
SOLVED A precipitate of copper(II) hydroxide is formed in a reaction. What change will help to What Happens When Copper Ii Hydroxide Is Heated When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. When it is mixed with copper (ii) carbonate, it is greenish. Write a balanced equation for the formation of copper(ii). What Happens When Copper Ii Hydroxide Is Heated.
From chemistrybb.blogspot.com
chemistry 3.18 Describe Reversible Reactions such as the Dehydration of Hydrated Copper (II What Happens When Copper Ii Hydroxide Is Heated When it is mixed with copper (ii) carbonate, it is greenish. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. Sodium hydroxide precipitates copper (ii) hydroxide: As a result, hydroxide ion can displace water from the copper (ii). What Happens When Copper Ii Hydroxide Is Heated.
From byjus.com
Detailed description of reaction— Heating of copper powder in presence of AIR What Happens When Copper Ii Hydroxide Is Heated As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. But isn't it a solid by itself too? It reacts with carbon dioxide to make copper (ii) carbonate when in air. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using. What Happens When Copper Ii Hydroxide Is Heated.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation, free download ID35476 What Happens When Copper Ii Hydroxide Is Heated Sodium hydroxide precipitates copper (ii) hydroxide: But isn't it a solid by itself too? As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When copper(ii) ions react with hydroxide ions in solution, they form the solid,. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
Making Copper Hydroxide YouTube What Happens When Copper Ii Hydroxide Is Heated But isn't it a solid by itself too? When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. It reacts with carbon dioxide to make copper (ii) carbonate when in air. So. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
How to write the formula for copper (II) hydroxide YouTube What Happens When Copper Ii Hydroxide Is Heated But isn't it a solid by itself too? Sodium hydroxide precipitates copper (ii) hydroxide: As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. When it is mixed with copper (ii) carbonate, it is greenish. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate. What Happens When Copper Ii Hydroxide Is Heated.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint What Happens When Copper Ii Hydroxide Is Heated It reacts with carbon dioxide to make copper (ii) carbonate when in air. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a. What Happens When Copper Ii Hydroxide Is Heated.
From woelen.homescience.net
Science made alive Chemistry/Solutions What Happens When Copper Ii Hydroxide Is Heated When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. Sodium hydroxide precipitates copper (ii) hydroxide: When it is mixed with copper (ii) carbonate, it is greenish. As a result, hydroxide ion. What Happens When Copper Ii Hydroxide Is Heated.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint What Happens When Copper Ii Hydroxide Is Heated Sodium hydroxide precipitates copper (ii) hydroxide: As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. But isn't it a solid by itself too? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When copper(ii) ions react with hydroxide ions in solution, they form the solid,. What Happens When Copper Ii Hydroxide Is Heated.
From www.slideshare.net
Action of heat on chemical compound e book What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When it is mixed with copper (ii) carbonate, it is greenish. But isn't it a solid by itself too? Sodium hydroxide precipitates copper (ii) hydroxide: When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Sodium hydroxide precipitates copper (ii) hydroxide: But isn't it a solid by itself too? When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate. What Happens When Copper Ii Hydroxide Is Heated.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation, free download ID35476 What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. But isn't it a solid by itself too? Sodium hydroxide precipitates copper (ii) hydroxide: It reacts with carbon dioxide to make copper. What Happens When Copper Ii Hydroxide Is Heated.
From vanittaran.weebly.com
Synthesis of copper(II) hydroxide ACQUIRE What Happens When Copper Ii Hydroxide Is Heated It reacts with carbon dioxide to make copper (ii) carbonate when in air. When it is mixed with copper (ii) carbonate, it is greenish. Sodium hydroxide precipitates copper (ii) hydroxide: But isn't it a solid by itself too? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When copper(ii) ions react with hydroxide ions in solution, they form. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
Heating a mixture of copper (II) oxide and carbon C0118 YouTube What Happens When Copper Ii Hydroxide Is Heated When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Sodium hydroxide precipitates copper (ii) hydroxide: When it is. What Happens When Copper Ii Hydroxide Is Heated.
From fphoto.photoshelter.com
science chemical reaction equilibrium cupric carbonate Fundamental Photographs The Art of What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When it is mixed with copper (ii) carbonate, it is greenish. But isn't it a solid by itself too? As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue. What Happens When Copper Ii Hydroxide Is Heated.
From hxeddpfhv.blob.core.windows.net
Copper Hydroxide State at Madeline Sumner blog What Happens When Copper Ii Hydroxide Is Heated But isn't it a solid by itself too? Sodium hydroxide precipitates copper (ii) hydroxide: When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When it is mixed with copper (ii) carbonate,. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
4_Copper(II) hydroxide + Heat YouTube What Happens When Copper Ii Hydroxide Is Heated As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. Sodium hydroxide precipitates copper (ii) hydroxide: But isn't it a solid by itself too? It reacts with carbon dioxide to make copper (ii) carbonate when in air. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated.. What Happens When Copper Ii Hydroxide Is Heated.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here What Happens When Copper Ii Hydroxide Is Heated So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Sodium hydroxide precipitates copper (ii) hydroxide: It reacts with carbon dioxide to make copper (ii) carbonate when in air. Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. When copper(ii) ions react with hydroxide ions in solution, they. What Happens When Copper Ii Hydroxide Is Heated.
From www.numerade.com
When copper(II) oxide is heated in the presence of hydrogen gas, elemental copper and water are What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. But isn't it a solid by itself too? When copper(ii) ions react with hydroxide ions in solution, they. What Happens When Copper Ii Hydroxide Is Heated.
From www.slideserve.com
PPT Polyatomic Ions and Their Compounds PowerPoint Presentation ID153488 What Happens When Copper Ii Hydroxide Is Heated Sodium hydroxide precipitates copper (ii) hydroxide: So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. It reacts with carbon dioxide to make copper (ii) carbonate when in air. As a result, hydroxide ion can displace water from the copper (ii). What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
Action of heat on copper (II) carbonate YouTube What Happens When Copper Ii Hydroxide Is Heated Write a balanced equation for the formation of copper(ii) hydroxide from copper(ii) nitrate and sodium hydroxide, using a metathesis (double. But isn't it a solid by itself too? When it is mixed with copper (ii) carbonate, it is greenish. It reacts with carbon dioxide to make copper (ii) carbonate when in air. When copper(ii) ions react with hydroxide ions in. What Happens When Copper Ii Hydroxide Is Heated.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II oxide YouTube What Happens When Copper Ii Hydroxide Is Heated It reacts with carbon dioxide to make copper (ii) carbonate when in air. When it is mixed with copper (ii) carbonate, it is greenish. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When copper(ii) ions react with hydroxide ions in solution, they form the solid, blue precipitate of copper(ii) hydroxide. Sodium hydroxide precipitates copper (ii) hydroxide: As. What Happens When Copper Ii Hydroxide Is Heated.