Condensing Definition In Chemistry . This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. When gas molecules transfer their. in other words, δhvap = − δhcond. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. condensation is the change in the state of matter from the gas phase to the liquid phase. condensation is the process by which a substance changes from a vapour state to a liquid state. A condensation reaction is also known as a dehydration reaction.
from general.chemistrysteps.com
a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. When gas molecules transfer their. condensation is the change in the state of matter from the gas phase to the liquid phase. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. in other words, δhvap = − δhcond. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. condensation is the process by which a substance changes from a vapour state to a liquid state. A condensation reaction is also known as a dehydration reaction. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of.
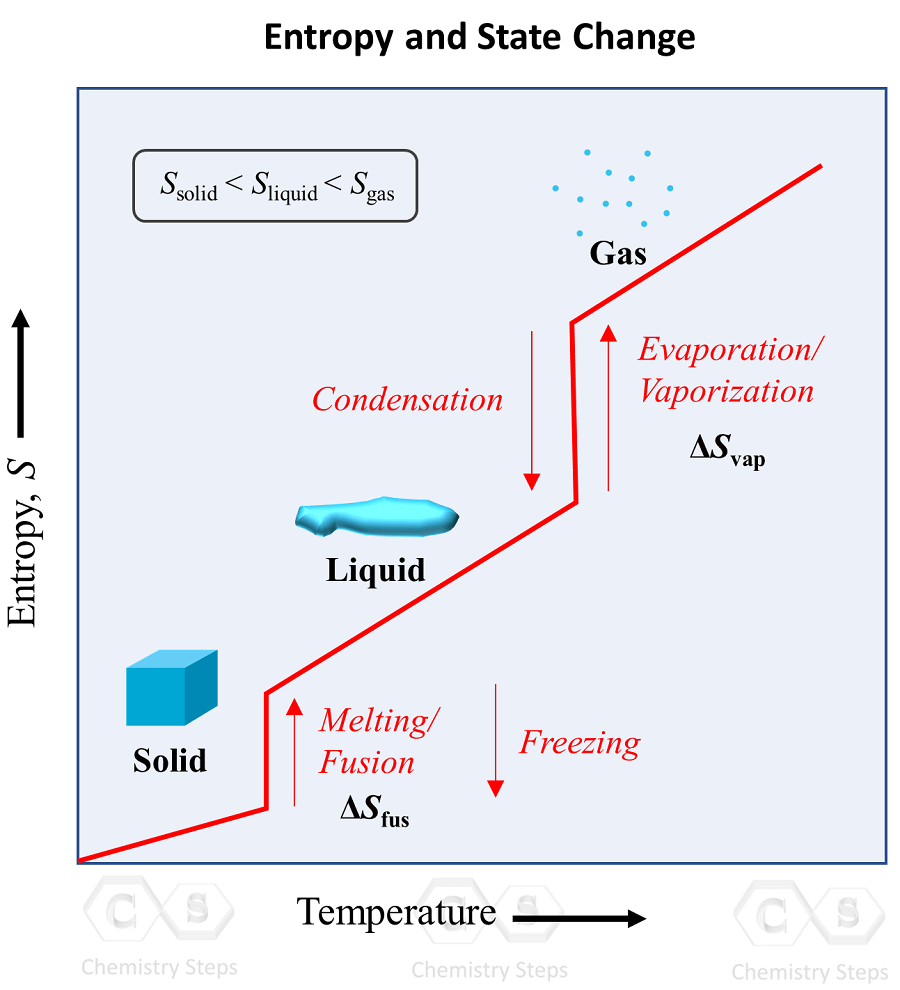
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps
Condensing Definition In Chemistry condensation is the change in the state of matter from the gas phase to the liquid phase. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. in other words, δhvap = − δhcond. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. condensation is the process by which a substance changes from a vapour state to a liquid state. A condensation reaction is also known as a dehydration reaction. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. condensation is the change in the state of matter from the gas phase to the liquid phase. When gas molecules transfer their.
From www.aakash.ac.in
Condensation in Chemistry Definition, Types and Importance of Condensing Definition In Chemistry a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. condensation is the process by which a substance changes from a vapour state to a liquid state. . Condensing Definition In Chemistry.
From sciencenotes.org
Condensation Reaction Definition and Examples Condensing Definition In Chemistry When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic. Condensing Definition In Chemistry.
From sciencespot1.weebly.com
Term 1 Science SpotMrs. MacCaul Condensing Definition In Chemistry condensation is the process by which a substance changes from a vapour state to a liquid state. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. A condensation reaction is also known as a dehydration reaction. a condensation reaction is a chemical reaction between two compounds where one of the products is water,. Condensing Definition In Chemistry.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Condensing Definition In Chemistry a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. condensation is the process by which a substance changes from a vapour state to a liquid state. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. This type of reaction forms an addition product and. Condensing Definition In Chemistry.
From www.slideserve.com
PPT Changes in State of Matter PowerPoint Presentation, free download Condensing Definition In Chemistry condensation is the process in which molecules of a gas slow down, come together, and form a liquid. condensation is the process by which a substance changes from a vapour state to a liquid state. in other words, δhvap = − δhcond. When gas molecules transfer their. a condensation reaction is a chemical reaction between two. Condensing Definition In Chemistry.
From www.alamy.com
Scientific illustration, diagram, howto art work, showing changing Condensing Definition In Chemistry When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. condensation is the process by which a substance changes from a vapour state to a liquid state. When gas molecules transfer their. in other words, δhvap = − δhcond. condensation is the change in the state of matter from the gas phase to. Condensing Definition In Chemistry.
From dewwool.com
Condensation DewWool Condensing Definition In Chemistry A condensation reaction is also known as a dehydration reaction. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. When gas molecules transfer their. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or. Condensing Definition In Chemistry.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Condensing Definition In Chemistry When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. When gas molecules transfer their. condensation is the change in the state of matter from the gas phase to the liquid phase. a condensation reaction. Condensing Definition In Chemistry.
From www.thoughtco.com
Aqueous Solution Definition in Chemistry Condensing Definition In Chemistry a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. This type of reaction forms an addition product and water in the presence of a catalyst. Condensing Definition In Chemistry.
From www.expii.com
Condensation — Definition & Overview Expii Condensing Definition In Chemistry condensation is the change in the state of matter from the gas phase to the liquid phase. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. . Condensing Definition In Chemistry.
From www.slideserve.com
PPT Cool Condensation PowerPoint Presentation, free download ID2835918 Condensing Definition In Chemistry condensation is the change in the state of matter from the gas phase to the liquid phase. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. in other words, δhvap = − δhcond. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. . Condensing Definition In Chemistry.
From exoodigxo.blob.core.windows.net
Condensing Meaning Dictionary at Richard Zigler blog Condensing Definition In Chemistry When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. condensation is the process by which a substance changes from a vapour state to a liquid state. condensation is the change in the state of matter from the gas phase to the liquid phase. a condensation reaction combines two molecules into one, releasing. Condensing Definition In Chemistry.
From www.worksheetsplanet.com
What is Chemistry Definition of Chemistry Condensing Definition In Chemistry condensation is the change in the state of matter from the gas phase to the liquid phase. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. When gas molecules transfer their. in other words, δhvap = − δhcond. condensation is the process in which molecules of a gas slow down, come together,. Condensing Definition In Chemistry.
From clipground.com
Condensation clipart 20 free Cliparts Download images on Clipground 2024 Condensing Definition In Chemistry When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. a. Condensing Definition In Chemistry.
From www.studyiq.com
Evaporation and Condensation, Definition, Process, Types, Difference Condensing Definition In Chemistry condensation is the process by which a substance changes from a vapour state to a liquid state. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. This type of reaction forms an addition product and water in the presence of a catalyst or. Condensing Definition In Chemistry.
From chemistry.stackexchange.com
experimental chemistry Choosing the right condenser Chemistry Stack Condensing Definition In Chemistry condensation is the process by which a substance changes from a vapour state to a liquid state. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. a condensation reaction. Condensing Definition In Chemistry.
From definitionklw.blogspot.com
Definition Of Condensing In Chemistry DEFINITION KLW Condensing Definition In Chemistry in other words, δhvap = − δhcond. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. When gas molecules transfer their. A condensation reaction is also known as a dehydration reaction. condensation is the. Condensing Definition In Chemistry.
From sciencenotes.org
What Is a Mixture in Chemistry? Definition and Examples Condensing Definition In Chemistry a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. condensation is the process by which a substance changes from a vapour state to a liquid state. . Condensing Definition In Chemistry.
From www.pinterest.com
Distillation Easy Science Distillation, Science chemistry, Physics Condensing Definition In Chemistry condensation is the process by which a substance changes from a vapour state to a liquid state. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. A condensation reaction is also known as a dehydration reaction. in other words, δhvap = − δhcond. condensation is the change in the state of matter. Condensing Definition In Chemistry.
From www.pinterest.co.uk
Pin on Diagrams and Infographics Condensing Definition In Chemistry A condensation reaction is also known as a dehydration reaction. condensation is the change in the state of matter from the gas phase to the liquid phase. When gas molecules transfer their. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. in. Condensing Definition In Chemistry.
From www.youtube.com
Explaining Boiling,Evaporation and Condensing in Complete Chemistry Condensing Definition In Chemistry When gas molecules transfer their. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. A condensation reaction is also known as a dehydration reaction. condensation is the change in the state of matter from the. Condensing Definition In Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Condensation reaction Condensing Definition In Chemistry in other words, δhvap = − δhcond. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. condensation is the change in the state of matter from the gas phase to the liquid phase. a condensation reaction is a chemical reaction between two compounds where. Condensing Definition In Chemistry.
From ar.inspiredpencil.com
Evaporation Chemistry Condensing Definition In Chemistry This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol,. Condensing Definition In Chemistry.
From ar.inspiredpencil.com
Condensation Diagram Particles Condensing Definition In Chemistry condensation is the change in the state of matter from the gas phase to the liquid phase. condensation is the process by which a substance changes from a vapour state to a liquid state. When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. a condensation reaction is a chemical reaction between two. Condensing Definition In Chemistry.
From proper-cooking.info
Condensation Separating Mixtures Condensing Definition In Chemistry When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. When gas molecules transfer their. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. condensation is the change in the state of matter from the gas phase to the liquid phase. in other words,. Condensing Definition In Chemistry.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Condensing Definition In Chemistry This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. condensation is the change in the state of matter from the gas phase to the liquid phase. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. A. Condensing Definition In Chemistry.
From exostfunr.blob.core.windows.net
Condensing Meaning And Example at Karen Tate blog Condensing Definition In Chemistry When gas molecules transfer their. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. in other words, δhvap = − δhcond. condensation is the change in the state of matter from the gas phase to the liquid phase. condensation is the process by which a substance changes. Condensing Definition In Chemistry.
From thechemistrynotes.com
Solvent Definition, Types, Incredible Uses, Examples Condensing Definition In Chemistry A condensation reaction is also known as a dehydration reaction. When gas molecules transfer their. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide,. Condensing Definition In Chemistry.
From shotprofessional22.gitlab.io
Ideal Definition Of Chemistry Chemical Reactions And Equations Class 10 Condensing Definition In Chemistry a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. in other words, δhvap = − δhcond. condensation is the process by which a substance changes from a vapour state to a liquid state. a condensation reaction is a chemical reaction between two compounds where one of the. Condensing Definition In Chemistry.
From dizz.com
Steam Condensing Plant Definition, Components, FAQ's [PDF] Design Condensing Definition In Chemistry a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. A condensation reaction is also known as a dehydration reaction. When gas molecules transfer their. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. . Condensing Definition In Chemistry.
From www.pinterest.co.uk
Pin on Reactions Condensing Definition In Chemistry When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. in other words, δhvap = − δhcond. condensation is the change in the state of matter from the gas. Condensing Definition In Chemistry.
From www.worksheetsplanet.com
What is Condensation Definition of Condensation Condensing Definition In Chemistry condensation is the change in the state of matter from the gas phase to the liquid phase. When gas molecules transfer their. This type of reaction forms an addition product and water in the presence of a catalyst or under acidic or basic conditions. condensation is the process in which molecules of a gas slow down, come together,. Condensing Definition In Chemistry.
From www.aakash.ac.in
Condensation in Chemistry Definition, Types and Importance of Condensing Definition In Chemistry A condensation reaction is also known as a dehydration reaction. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. condensation is the change in the state of matter from the. Condensing Definition In Chemistry.
From www.worksheetsplanet.com
What is the Solvent? Condensing Definition In Chemistry condensation is the process by which a substance changes from a vapour state to a liquid state. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. in other words, δhvap = − δhcond. condensation is the change in the state of matter from the gas phase to. Condensing Definition In Chemistry.
From ceibjcxo.blob.core.windows.net
Condenser Types Definition at Matthew Gil blog Condensing Definition In Chemistry When 1mol of water at 100oc and 1atm pressure is converted to 1mol of. a condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. a condensation reaction combines two molecules into one, releasing a small molecule such as water in the process. This type. Condensing Definition In Chemistry.