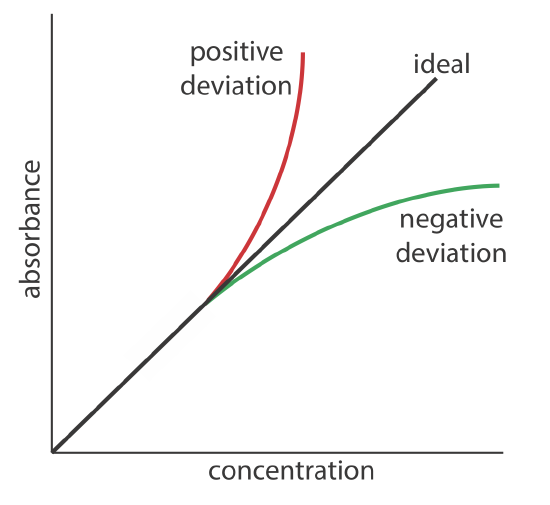
Beer's Law From Graph . The direct relationship between absorbance and concentration for a solution is known as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Use the beer's law plot to determine the concentration of an unknown analyte. The concentration of an unknown niso4. It is also referred to as beer’s law.
from chem.libretexts.org
As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The concentration of an unknown niso4. Use the beer's law plot to determine the concentration of an unknown analyte. It is also referred to as beer’s law.
8.2 Beer's Law Chemistry LibreTexts
Beer's Law From Graph It is also referred to as beer’s law. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Use the beer's law plot to determine the concentration of an unknown analyte. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). It is also referred to as beer’s law.
From chart-studio.plotly.com
Beer's Law (Blue Dye Absorbance vs. Concentration) scatter chart Beer's Law From Graph Use the beer's law plot to determine the concentration of an unknown analyte. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The direct relationship between absorbance and concentration for a solution is known as beer’s law. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a. Beer's Law From Graph.
From lelandchemclub.weebly.com
Solutions Leland Chemistry Club Beer's Law From Graph Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The direct relationship between absorbance and concentration for a solution is known as beer’s law. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. It is also referred to as beer’s law. Calculate the concentration of. Beer's Law From Graph.
From chart-studio.plotly.com
Beer's Law Plot scatter chart made by Java789 plotly Beer's Law From Graph It is also referred to as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The concentration of an. Beer's Law From Graph.
From chart-studio.plotly.com
Beer's Law (Absorbance vs. Molar Concentration) scatter chart made by Beer's Law From Graph It is also referred to as beer’s law. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Use the beer's law plot to determine the concentration of an unknown analyte. Since the concentration, path length and molar absorptivity are all directly. Beer's Law From Graph.
From www.youtube.com
Beer's law Excel Plot Analysis of Unknown Solution Concentrations YouTube Beer's Law From Graph The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The concentration of an unknown niso4. Since the concentration, path length and molar absorptivity are all directly proportional. Beer's Law From Graph.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer's Law From Graph Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The concentration of an unknown niso4. It is also referred to as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Use the beer's law plot to determine the concentration of an unknown analyte. Beer's law forms the basis. Beer's Law From Graph.
From www.chegg.com
Beer’s law (A= ECl) is a linear equation of the form Beer's Law From Graph Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. It is also referred to as beer’s law. Use the beer's law. Beer's Law From Graph.
From chart-studio.plotly.com
Beer's Law Plot for FeSCN2+ scatter chart made by Nelsonr462 plotly Beer's Law From Graph Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Use the beer's law plot to determine the concentration of an unknown analyte. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s. Beer's Law From Graph.
From klawmisjt.blob.core.windows.net
Beer's Law Plot Excel at Harold Osborne blog Beer's Law From Graph As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. Use the beer's law plot to determine the concentration of an unknown analyte. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law From Graph.
From loericfgn.blob.core.windows.net
Beer's Law Values at Alfred Nee blog Beer's Law From Graph Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. It is also referred to as beer’s law. Since the concentration, path. Beer's Law From Graph.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law From Graph Use the beer's law plot to determine the concentration of an unknown analyte. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). It is also referred to as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The concentration of an unknown niso4.. Beer's Law From Graph.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law From Graph Use the beer's law plot to determine the concentration of an unknown analyte. The direct relationship between absorbance and concentration for a solution is known as beer’s law. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer's Law From Graph.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law From Graph The direct relationship between absorbance and concentration for a solution is known as beer’s law. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. It is also referred to as beer’s law. The concentration of an unknown niso4. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer's Law From Graph.
From www.chem.ucla.edu
Beer's Law Tutorial Beer's Law From Graph Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Use the beer's law plot to determine the concentration of an unknown analyte.. Beer's Law From Graph.
From loericfgn.blob.core.windows.net
Beer's Law Values at Alfred Nee blog Beer's Law From Graph Use the beer's law plot to determine the concentration of an unknown analyte. It is also referred to as beer’s law. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a. Beer's Law From Graph.
From www.chegg.com
Solved The Beer’s Law plot is one of the most useful for a Beer's Law From Graph Use the beer's law plot to determine the concentration of an unknown analyte. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations.. Beer's Law From Graph.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law From Graph As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. It is also referred to as beer’s law. The concentration of an unknown niso4. Use the beer's law plot to. Beer's Law From Graph.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer's Law From Graph As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The concentration of an unknown niso4. Calculate the concentration of a dilute. Beer's Law From Graph.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law From Graph The direct relationship between absorbance and concentration for a solution is known as beer’s law. It is also referred to as beer’s law. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The concentration of an. Beer's Law From Graph.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law From Graph Use the beer's law plot to determine the concentration of an unknown analyte. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. The concentration of an unknown niso4. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The. Beer's Law From Graph.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law From Graph Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given.. Beer's Law From Graph.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law From Graph As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. It is also referred to as beer’s law. Use the beer's law plot to determine the concentration of an unknown analyte. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. Since the concentration, path length. Beer's Law From Graph.
From www.scribd.com
Beer S Law Plot of KMnO4 PDF PDF Beer's Law From Graph It is also referred to as beer’s law. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Use the beer's law plot to determine the concentration of an unknown analyte. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law From Graph.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law From Graph It is also referred to as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The concentration of an unknown niso4. Use the beer's law plot to determine the concentration of an. Beer's Law From Graph.
From www.slideserve.com
PPT Spectrophotometric Determination of Iron Using 1,10 Beer's Law From Graph Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Use the beer's law plot to determine the concentration of an unknown analyte. The concentration of an unknown niso4. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the. Beer's Law From Graph.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer's Law From Graph Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Use the beer's law plot to determine the concentration of an unknown analyte. It is also referred to as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional. Beer's Law From Graph.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer's Law From Graph It is also referred to as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The concentration of. Beer's Law From Graph.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer's Law From Graph As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. Since the concentration, path length. Beer's Law From Graph.
From www.researchgate.net
Beer's law plot of second derivative data [HMBATSC] = 1 x 103 M Beer's Law From Graph The direct relationship between absorbance and concentration for a solution is known as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It is also referred to as beer’s law. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Beer's law forms the. Beer's Law From Graph.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry Beer's Law From Graph Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). It is also referred to as beer’s law. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law forms the basis. Beer's Law From Graph.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer's Law From Graph Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. It is also referred to as beer’s law. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The direct relationship between absorbance and concentration for a solution is known. Beer's Law From Graph.
From www.researchgate.net
Calibration curve according to BeerLambert equation for tetracycline Beer's Law From Graph Beer's law forms the basis for the analytical use of spectroscopy to determine concentrations. The direct relationship between absorbance and concentration for a solution is known as beer’s law. It is also referred to as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Calculate the concentration. Beer's Law From Graph.
From www.slideserve.com
PPT chapter 2 PowerPoint Presentation, free download ID540228 Beer's Law From Graph Use the beer's law plot to determine the concentration of an unknown analyte. The concentration of an unknown niso4. As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law From Graph.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law From Graph As indicated by equation \(\pageindex{3}\), a plot of the absorbance at a given. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Use the beer's law plot to determine the concentration of an unknown analyte. The direct relationship between absorbance and concentration for a solution is known as beer’s law. It is also referred to as. Beer's Law From Graph.
From royalsocietypublishing.org
Misuse of BeerLambert Law and other calibration curves Royal Society Beer's Law From Graph The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It is also referred to as beer’s law. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy. Beer's Law From Graph.