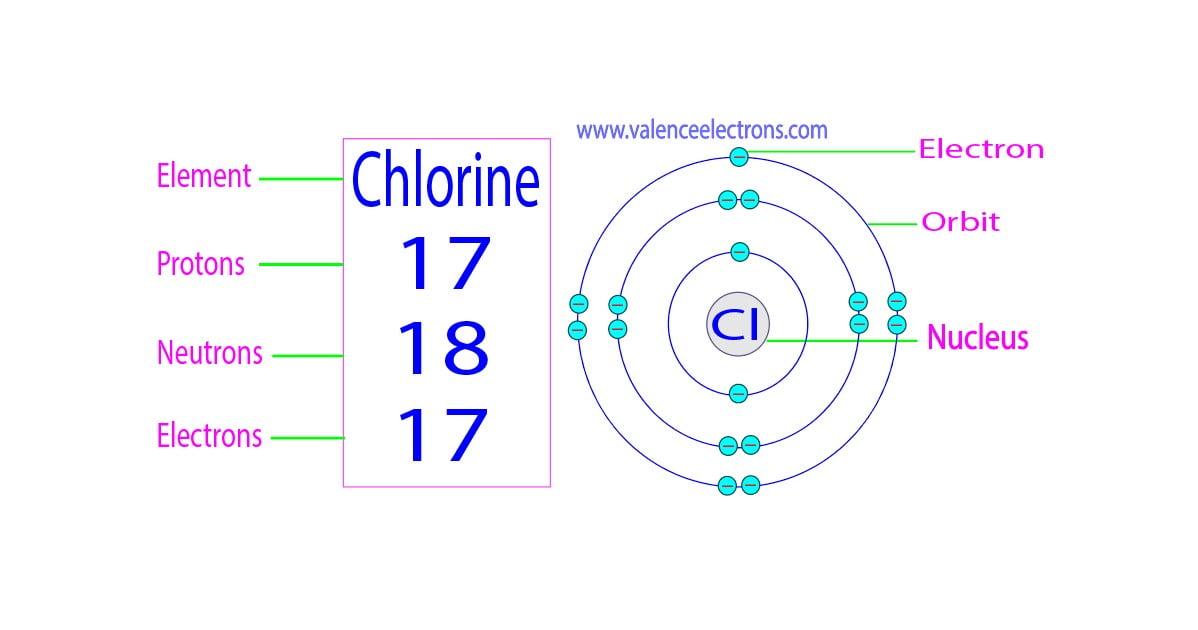
Valence Electrons Chlorine Ion . 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Valence electrons are the number of electrons present in the outermost shell of an atom. This makes chlorine a cl−1. But for most of the transition. A neutral chlorine atom has seven electrons in its outermost shell. Knowing how to find the number of valence electrons in. It needs one electron to make it stable at 8 electrons in its valence shells. The last shell of a chlorine atom has 7. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Chlorine has 7 valence electrons.
from loeylrncp.blob.core.windows.net
The last shell of a chlorine atom has 7. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Knowing how to find the number of valence electrons in. Valence electrons are the number of electrons present in the outermost shell of an atom. A neutral chlorine atom has seven electrons in its outermost shell. It needs one electron to make it stable at 8 electrons in its valence shells. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine has 7 valence electrons. This makes chlorine a cl−1.
Chlorine Electrons And Protons at Edward Thompson blog
Valence Electrons Chlorine Ion Knowing how to find the number of valence electrons in. Valence electrons are the number of electrons present in the outermost shell of an atom. It needs one electron to make it stable at 8 electrons in its valence shells. This makes chlorine a cl−1. Chlorine has 7 valence electrons. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Only one more electron is needed to achieve an octet in chlorine’s valence shell. A neutral chlorine atom has seven electrons in its outermost shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Knowing how to find the number of valence electrons in. But for most of the transition. The last shell of a chlorine atom has 7.
From animalia-life.club
Lewis Dot Structure For Chlorine Valence Electrons Chlorine Ion The last shell of a chlorine atom has 7. It needs one electron to make it stable at 8 electrons in its valence shells. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Only one more electron is needed to achieve an octet in chlorine’s. Valence Electrons Chlorine Ion.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. Valence electrons are the number of electrons present in the outermost shell of an atom. The last shell of a chlorine atom has 7. It needs one electron to make it stable at 8 electrons in its valence shells. In chemistry, valence electrons are the electrons that are located. Valence Electrons Chlorine Ion.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Valence Electrons Chlorine Ion Knowing how to find the number of valence electrons in. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. It needs one electron to make it stable at 8 electrons in its valence shells. But for most of the transition. In chemistry, valence electrons are. Valence Electrons Chlorine Ion.
From slideplayer.com
Chapter 19 Molecules and Compounds ppt download Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. The last shell of a chlorine atom has 7. It needs one electron to make it stable at 8 electrons in its valence shells. Knowing how to find the number of valence electrons in. Valence electrons are the number of electrons present in the outermost shell of an atom.. Valence Electrons Chlorine Ion.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Valence Electrons Chlorine Ion It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine has 7 valence electrons. The last shell of a chlorine atom has 7. Valence electrons are the number of electrons present in the outermost shell of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Valence Electrons Chlorine Ion.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Valence Electrons Chlorine Ion Valence electrons are the number of electrons present in the outermost shell of an atom. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. This makes chlorine a cl−1. Chlorine has 7 valence electrons. A neutral chlorine atom has seven electrons in its outermost shell. It needs one electron to make. Valence Electrons Chlorine Ion.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Valence Electrons Chlorine Ion 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Knowing how to find the number of valence electrons in. Chlorine has 7 valence electrons. But for most of the transition. Only one more electron is needed to achieve an octet in chlorine’s valence shell. In. Valence Electrons Chlorine Ion.
From brokeasshome.com
Periodic Table Chlorine Electrons Valence Electrons Chlorine Ion But for most of the transition. The last shell of a chlorine atom has 7. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. Valence electrons are the number of electrons present in the outermost shell of an atom. 93 rows you may assume the valences of the chemical. Valence Electrons Chlorine Ion.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Valence Electrons Chlorine Ion Only one more electron is needed to achieve an octet in chlorine’s valence shell. Knowing how to find the number of valence electrons in. The last shell of a chlorine atom has 7. It needs one electron to make it stable at 8 electrons in its valence shells. In chemistry, valence electrons are the electrons that are located in the. Valence Electrons Chlorine Ion.
From www.ck12.org
Valence Electrons CK12 Foundation Valence Electrons Chlorine Ion Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. This makes chlorine a cl−1. Chlorine has 7 valence electrons. But for most of the transition. It needs one electron to make. Valence Electrons Chlorine Ion.
From kunduz.com
[ANSWERED] The electron dot diagram for a neutral at... Physical Valence Electrons Chlorine Ion In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Knowing how to find the number of valence electrons in. The last shell of a chlorine atom has 7. Chlorine has 7 valence electrons. This makes chlorine a cl−1. 93 rows you may assume the valences of the chemical elements—the number of. Valence Electrons Chlorine Ion.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Valence Electrons Chlorine Ion Only one more electron is needed to achieve an octet in chlorine’s valence shell. The last shell of a chlorine atom has 7. It needs one electron to make it stable at 8 electrons in its valence shells. A neutral chlorine atom has seven electrons in its outermost shell. Valence electrons are the number of electrons present in the outermost. Valence Electrons Chlorine Ion.
From sciencenotes.org
Chlorine Facts Valence Electrons Chlorine Ion Knowing how to find the number of valence electrons in. Chlorine has 7 valence electrons. But for most of the transition. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Valence electrons are the number of electrons present in the outermost shell of an atom. The last shell of a chlorine. Valence Electrons Chlorine Ion.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Valence Electrons Chlorine Ion Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. A neutral chlorine atom has seven electrons in its outermost shell. Knowing how to find the number of valence electrons in. This. Valence Electrons Chlorine Ion.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. Chlorine has 7 valence electrons. Knowing how to find the number of valence electrons in. It needs one electron to make it stable at 8 electrons in its valence shells. This makes chlorine a cl−1. 93 rows you may assume the valences of the chemical elements—the number of electrons. Valence Electrons Chlorine Ion.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model Valence Electrons Chlorine Ion The last shell of a chlorine atom has 7. Knowing how to find the number of valence electrons in. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Only one more. Valence Electrons Chlorine Ion.
From www.slideserve.com
PPT Electron Energy Levels and Configurations PowerPoint Presentation Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. This makes chlorine a cl−1. Knowing how to find the number of valence electrons in. Valence electrons are the number of electrons present in the outermost shell of an atom. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. It. Valence Electrons Chlorine Ion.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Valence Electrons Chlorine Ion But for most of the transition. Valence electrons are the number of electrons present in the outermost shell of an atom. Knowing how to find the number of valence electrons in. A neutral chlorine atom has seven electrons in its outermost shell. It needs one electron to make it stable at 8 electrons in its valence shells. In chemistry, valence. Valence Electrons Chlorine Ion.
From pixels.com
Chlorine Electron Configuration Photograph by Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. Knowing how to find the number of valence electrons in. The last shell of a chlorine atom has 7. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Valence electrons are the number of electrons present in the outermost shell. Valence Electrons Chlorine Ion.
From chemtech-us.com
15 Interesting Facts About Chlorine Valence Electrons Chlorine Ion In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. But for most of the transition. The last shell of a chlorine atom has 7. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. This makes chlorine. Valence Electrons Chlorine Ion.
From www.youtube.com
Chlorine Electron Configuration YouTube Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons are the number of electrons present in the outermost shell of an atom. This makes chlorine a cl−1. The last shell of a. Valence Electrons Chlorine Ion.
From saylordotorg.github.io
Ions Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. Chlorine has 7 valence electrons. This makes chlorine a cl−1. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Knowing how to find the number of valence electrons in. The last shell of a chlorine atom has 7. It needs. Valence Electrons Chlorine Ion.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Valence Electrons Chlorine Ion But for most of the transition. Chlorine has 7 valence electrons. Knowing how to find the number of valence electrons in. A neutral chlorine atom has seven electrons in its outermost shell. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. 93 rows you may assume the valences of the chemical. Valence Electrons Chlorine Ion.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Valence Electrons Chlorine Ion This makes chlorine a cl−1. Only one more electron is needed to achieve an octet in chlorine’s valence shell. It needs one electron to make it stable at 8 electrons in its valence shells. Knowing how to find the number of valence electrons in. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Valence Electrons Chlorine Ion.
From valenceelectrons.com
How to Find the Valence Electrons for Chlorine (Cl)? Valence Electrons Chlorine Ion This makes chlorine a cl−1. Knowing how to find the number of valence electrons in. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Valence electrons are the number of electrons present in the outermost. Valence Electrons Chlorine Ion.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Valence Electrons Chlorine Ion 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. This makes chlorine a cl−1. A neutral chlorine atom has seven electrons in its outermost shell. It needs. Valence Electrons Chlorine Ion.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. It needs one electron to make it stable at 8 electrons in its valence shells. Knowing how to find the number of valence electrons in. This makes chlorine a cl−1. The last shell of a chlorine atom has 7. In chemistry, valence electrons are the electrons that are located. Valence Electrons Chlorine Ion.
From guide-scientific.com
¿Cuántos electrones de valencia tiene el Cloro (Cl)? Valencia de cloro. Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. This makes chlorine a cl−1. It needs one electron to make it stable at 8 electrons in its valence. Valence Electrons Chlorine Ion.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Valence Electrons Chlorine Ion Only one more electron is needed to achieve an octet in chlorine’s valence shell. Chlorine has 7 valence electrons. Knowing how to find the number of valence electrons in. Valence electrons are the number of electrons present in the outermost shell of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Valence Electrons Chlorine Ion.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Valence Electrons Chlorine Ion It needs one electron to make it stable at 8 electrons in its valence shells. Knowing how to find the number of valence electrons in. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. A neutral chlorine atom has seven electrons in its outermost shell.. Valence Electrons Chlorine Ion.
From www.teachoo.com
Ionic and Covalent Bonds Concepts Valence Electrons Chlorine Ion Valence electrons are the number of electrons present in the outermost shell of an atom. The last shell of a chlorine atom has 7. Chlorine has 7 valence electrons. A neutral chlorine atom has seven electrons in its outermost shell. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons. Valence Electrons Chlorine Ion.
From loeylrncp.blob.core.windows.net
Chlorine Electrons And Protons at Edward Thompson blog Valence Electrons Chlorine Ion 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. A neutral chlorine atom has seven electrons in its outermost shell. This makes chlorine a cl−1. But for most of the transition. Only one more electron is needed to achieve an octet in chlorine’s valence shell.. Valence Electrons Chlorine Ion.
From joiyaimcz.blob.core.windows.net
Chlorine Valence Shell Electron Configuration at Emma Colburn blog Valence Electrons Chlorine Ion But for most of the transition. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Chlorine has 7 valence electrons. The last shell of a chlorine atom has 7. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In chemistry,. Valence Electrons Chlorine Ion.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Valence Electrons Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. Valence electrons are the number of electrons present in the outermost shell of an atom. This makes chlorine a cl−1. Chlorine has 7 valence electrons. But for most of the transition. Only one more electron is needed to achieve an octet in chlorine’s valence shell. It needs one electron. Valence Electrons Chlorine Ion.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Valence Electrons Chlorine Ion In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. But for most of the transition. A neutral chlorine atom has seven electrons in its outermost shell. The last shell of a chlorine atom has 7. Chlorine has 7 valence electrons. This makes chlorine a cl−1. 93 rows you may assume the. Valence Electrons Chlorine Ion.