What Is A Calorimeter Quizlet . Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. What is the principle behind calorimetry? A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Apply the first law of thermodynamics to calorimetry. It uses devices called calorimeters, which measure the change in.
from www.chegg.com
Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. For example, when an exothermic. What is the principle behind calorimetry? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold.
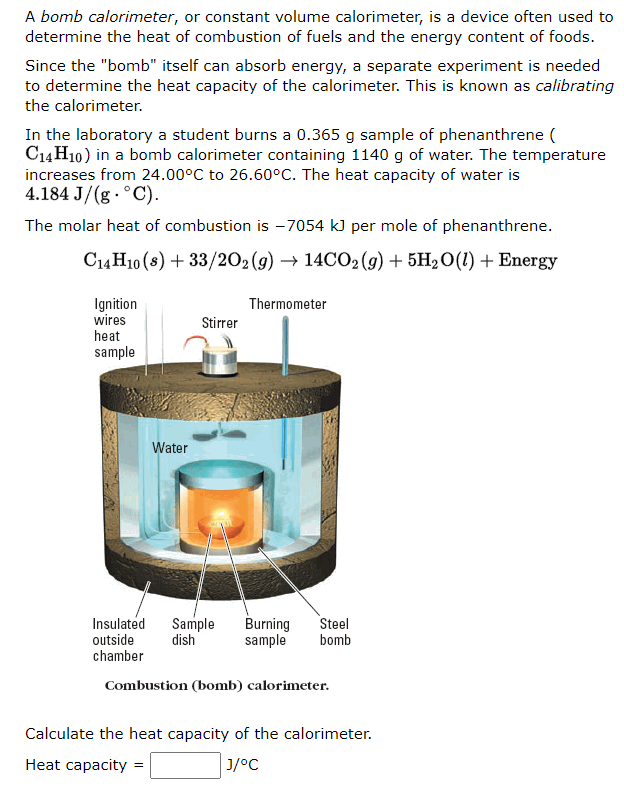
Solved A bomb calorimeter, or constant volume calorimeter,
What Is A Calorimeter Quizlet Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. It uses devices called calorimeters, which measure the change in. What is the principle behind calorimetry? For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.
From 2012books.lardbucket.org
Calorimetry What Is A Calorimeter Quizlet What is the principle behind calorimetry? Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses. What Is A Calorimeter Quizlet.
From quizlet.com
Calorimetry (When to use the equations) Diagram Quizlet What Is A Calorimeter Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. It uses devices called calorimeters, which measure the change in. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure. What Is A Calorimeter Quizlet.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Is A Calorimeter Quizlet It uses devices called calorimeters, which measure the change in. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot. What Is A Calorimeter Quizlet.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First What Is A Calorimeter Quizlet What is the principle behind calorimetry? Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. What Is A Calorimeter Quizlet.
From www.youtube.com
050 Calorimetry YouTube What Is A Calorimeter Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure. What Is A Calorimeter Quizlet.
From scienceinfo.com
Calorimeter Definition, Types and Uses What Is A Calorimeter Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. Compare heat flow from hot to. What Is A Calorimeter Quizlet.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses What Is A Calorimeter Quizlet Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of. What Is A Calorimeter Quizlet.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is A Calorimeter Quizlet What is the principle behind calorimetry? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Study with quizlet. What Is A Calorimeter Quizlet.
From quizlet.com
Calorimeter Diagram Quizlet What Is A Calorimeter Quizlet Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in. What Is A Calorimeter Quizlet.
From cezvuytp.blob.core.windows.net
What Is A Bomb Calorimeter Quizlet at Earline Numbers blog What Is A Calorimeter Quizlet It uses devices called calorimeters, which measure the change in. Apply the first law of thermodynamics to calorimetry. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. Compare heat flow from hot to. What Is A Calorimeter Quizlet.
From chemistrytalk.org
Calorimetry ChemTalk What Is A Calorimeter Quizlet Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. It uses devices called calorimeters, which measure the change in. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical,. What Is A Calorimeter Quizlet.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture What Is A Calorimeter Quizlet Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the. What Is A Calorimeter Quizlet.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Is A Calorimeter Quizlet Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. What is the principle behind calorimetry? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the change in. A calorimeter is. What Is A Calorimeter Quizlet.
From byjus.com
The calorimeter is commonly made up of……..because it has……. What Is A Calorimeter Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Study with quizlet and memorize. What Is A Calorimeter Quizlet.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Is A Calorimeter Quizlet A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. It uses devices called calorimeters, which measure the change in. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. What is the principle behind calorimetry? Calorimeter, device for measuring the heat developed during a mechanical, electrical,. What Is A Calorimeter Quizlet.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Is A Calorimeter Quizlet For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Apply. What Is A Calorimeter Quizlet.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME What Is A Calorimeter Quizlet It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction. What Is A Calorimeter Quizlet.
From quizlet.com
Sketch a simple calorimeter and label the purpose of each co Quizlet What Is A Calorimeter Quizlet It uses devices called calorimeters, which measure the change in. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. What Is A Calorimeter Quizlet.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 What Is A Calorimeter Quizlet For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. What is the principle behind calorimetry? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or. What Is A Calorimeter Quizlet.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure What Is A Calorimeter Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. It uses devices called calorimeters, which measure the change in. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. What Is A Calorimeter Quizlet.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Is A Calorimeter Quizlet A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Study with quizlet and memorize flashcards. What Is A Calorimeter Quizlet.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Is A Calorimeter Quizlet It uses devices called calorimeters, which measure the change in. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved. What Is A Calorimeter Quizlet.
From saylordotorg.github.io
Calorimetry What Is A Calorimeter Quizlet A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object. What Is A Calorimeter Quizlet.
From quizlet.com
Calorimeter Diagram Quizlet What Is A Calorimeter Quizlet A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. What is the principle. What Is A Calorimeter Quizlet.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume What Is A Calorimeter Quizlet It uses devices called calorimeters, which measure the change in. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the heat flow of a. What Is A Calorimeter Quizlet.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, What Is A Calorimeter Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Compare heat flow from hot to cold objects. What Is A Calorimeter Quizlet.
From cezvuytp.blob.core.windows.net
What Is A Bomb Calorimeter Quizlet at Earline Numbers blog What Is A Calorimeter Quizlet It uses devices called calorimeters, which measure the change in. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. A calorimeter is a device used to measure the heat flow of a chemical reaction. What Is A Calorimeter Quizlet.
From cezvuytp.blob.core.windows.net
What Is A Bomb Calorimeter Quizlet at Earline Numbers blog What Is A Calorimeter Quizlet For example, when an exothermic. What is the principle behind calorimetry? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with a cold. Calorimetry is the set of techniques used to. What Is A Calorimeter Quizlet.
From cezvuytp.blob.core.windows.net
What Is A Bomb Calorimeter Quizlet at Earline Numbers blog What Is A Calorimeter Quizlet What is the principle behind calorimetry? It uses devices called calorimeters, which measure the change in. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Study with quizlet and memorize flashcards containing terms like calorimetry:, heat:, when a hot object is placed in contact with. What Is A Calorimeter Quizlet.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is A Calorimeter Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. What Is A Calorimeter Quizlet.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 What Is A Calorimeter Quizlet Apply the first law of thermodynamics to calorimetry. What is the principle behind calorimetry? Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the change in. Study with. What Is A Calorimeter Quizlet.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement What Is A Calorimeter Quizlet For example, when an exothermic. It uses devices called calorimeters, which measure the change in. Study with quizlet and memorize flashcards terms like thermochemistry, calorimeter, reaction chamber and more. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the. What Is A Calorimeter Quizlet.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure What Is A Calorimeter Quizlet Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. What Is A Calorimeter Quizlet.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Is A Calorimeter Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Compare heat flow from hot to cold objects in. What Is A Calorimeter Quizlet.
From quizlet.com
Investigate the characteristics of a professionallydesigned Quizlet What Is A Calorimeter Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. What. What Is A Calorimeter Quizlet.