Titration Calculations Equation . Ma is the molarity of the acid, while mb is the molarity of the base. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Find the number of moles of acid. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Hcl (aq) + naoh (aq) → nacl (aq) + h 2 o. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. In the naoh—ch 3 cooh reaction eq. Ma × va = mb × vb. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. How to do titration calculations. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Va and vb are the volumes of the acid. Moles of acid = concentration x volume in dm 3. Deduce the number of moles. Calculate the amount of sodium hydroxide in moles.
from www.youtube.com
Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Moles of acid = concentration x volume in dm 3. Calculate the amount of sodium hydroxide in moles. Once a titration is completed and the average titre has been calculated, you can. In the naoh—ch 3 cooh reaction eq. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Ma × va = mb × vb.
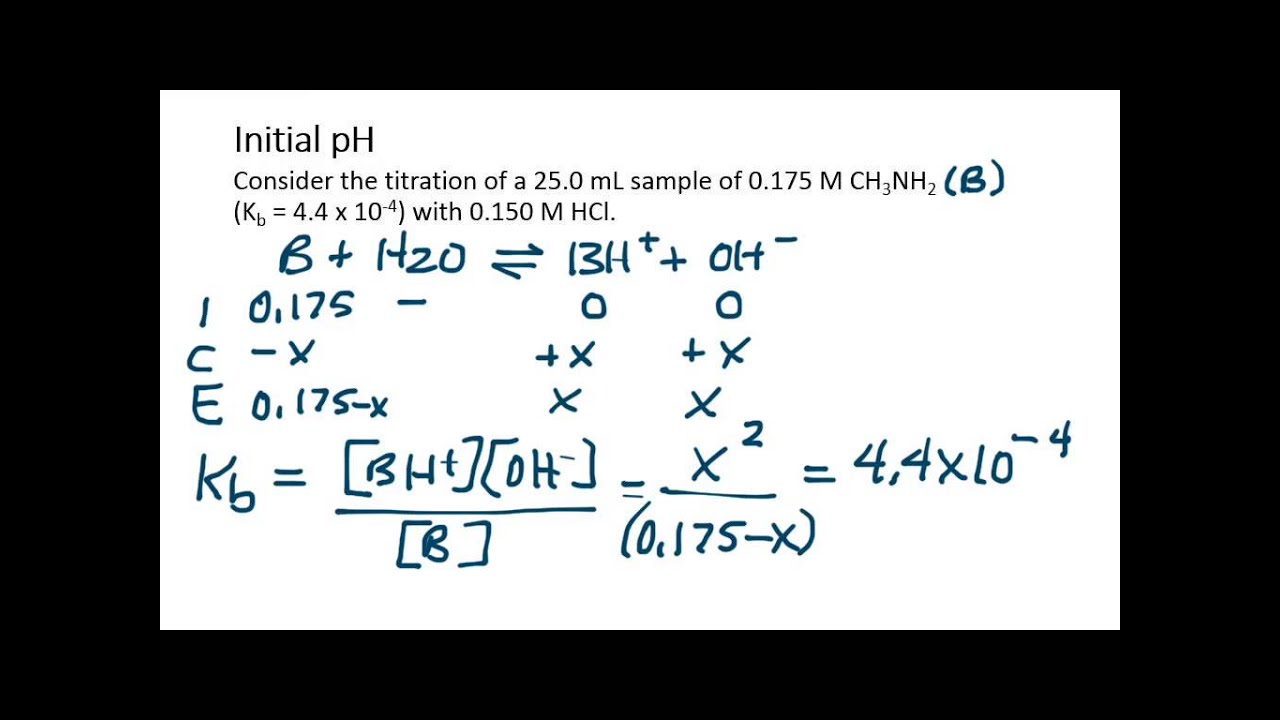
Calculations for Titration of Weak Base with Strong Acid, initial pH
Titration Calculations Equation Moles of acid = concentration x volume in dm 3. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Find the number of moles of acid. Ma is the molarity of the acid, while mb is the molarity of the base. Moles of acid = concentration x volume in dm 3. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. Ma × va = mb × vb. Va and vb are the volumes of the acid. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Calculate the amount of sodium hydroxide in moles. Once a titration is completed and the average titre has been calculated, you can. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. How to do titration calculations. Deduce the number of moles. In the naoh—ch 3 cooh reaction eq.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Titration Calculations Equation Ma × va = mb × vb. Calculate the amount of sodium hydroxide in moles. In the naoh—ch 3 cooh reaction eq. How to do titration calculations. Find the number of moles of acid. Ma is the molarity of the acid, while mb is the molarity of the base. Moles of acid = concentration x volume in dm 3. The. Titration Calculations Equation.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Calculations Equation How to do titration calculations. Moles of acid = concentration x volume in dm 3. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Ma × va = mb ×. Titration Calculations Equation.
From www.youtube.com
Titration Calculations YouTube Titration Calculations Equation Moles of acid = concentration x volume in dm 3. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Find the number of moles of acid. How to do titration. Titration Calculations Equation.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Calculations Equation Ma is the molarity of the acid, while mb is the molarity of the base. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Ma × va = mb × vb. Hcl (aq) + naoh (aq) → nacl (aq) + h 2 o. Va and vb are the volumes. Titration Calculations Equation.
From www.youtube.com
Total Alkalinity Titration Method and Calculations YouTube Titration Calculations Equation In the naoh—ch 3 cooh reaction eq. Ma is the molarity of the acid, while mb is the molarity of the base. Moles of acid = concentration x volume in dm 3. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. A titration is used to find an unknown concentration of a solution by reacting it. Titration Calculations Equation.
From meetingtarget11.gitlab.io
Amazing Youtube Titration Calculations Balancing Chemical Equations Titration Calculations Equation The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Va and vb are the volumes of the acid. Hcl (aq) + naoh (aq) → nacl (aq) + h 2 o. Deduce the number of moles. The example below demonstrates the technique to solve a titration. Titration Calculations Equation.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Calculations Equation Moles of acid = concentration x volume in dm 3. Va and vb are the volumes of the acid. How to do titration calculations. Once a titration is completed and the average titre has been calculated, you can. Calculate the amount of sodium hydroxide in moles. A titration is used to find an unknown concentration of a solution by reacting. Titration Calculations Equation.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Equation Ma is the molarity of the acid, while mb is the molarity of the base. Once a titration is completed and the average titre has been calculated, you can. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. How to do titration calculations. In the naoh—ch 3 cooh reaction eq.. Titration Calculations Equation.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Calculations Equation Moles of acid = concentration x volume in dm 3. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Hcl (aq) + naoh (aq) → nacl (aq) + h 2 o. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. The example below demonstrates the technique. Titration Calculations Equation.
From www.youtube.com
Calculations for Titration of Weak Base with Strong Acid, initial pH Titration Calculations Equation Moles of acid = concentration x volume in dm 3. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Ma × va = mb × vb. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Deduce the number of moles. The. Titration Calculations Equation.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Calculations Equation The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Ma × va = mb × vb. How to do titration calculations. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Calculate the amount. Titration Calculations Equation.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Calculations Equation In the naoh—ch 3 cooh reaction eq. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio. Titration Calculations Equation.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Calculations Equation In the naoh—ch 3 cooh reaction eq. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Va and vb are the volumes of the acid. Ma × va. Titration Calculations Equation.
From chemistry.stackexchange.com
homework How to calculate the dissociation constant of a weak acid Titration Calculations Equation Va and vb are the volumes of the acid. Deduce the number of moles. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Once a titration is completed and the average titre has been calculated, you can. Moles of acid = concentration x volume in dm 3. Calculate the. Titration Calculations Equation.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Titration Calculations Equation A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Calculate the amount of sodium hydroxide in moles. Moles of acid = concentration x volume in dm 3. Once a titration is completed and the average titre has been calculated, you can. How to do titration calculations. The example. Titration Calculations Equation.
From ar.inspiredpencil.com
Titration Equation Titration Calculations Equation How to do titration calculations. Va and vb are the volumes of the acid. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In the naoh—ch 3 cooh reaction eq. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. Titration Calculations Equation.
From pdfslide.net
(PDF) Acid and Base Titrations Equation Guide · Page I11 / Titration Titration Calculations Equation Calculate the amount of sodium hydroxide in moles. Find the number of moles of acid. Moles of acid = concentration x volume in dm 3. In the naoh—ch 3 cooh reaction eq. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. When hydrochloric acid is. Titration Calculations Equation.
From www.studypool.com
SOLUTION Titration calculations and answers Studypool Titration Calculations Equation Once a titration is completed and the average titre has been calculated, you can. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Calculate the amount of sodium hydroxide in moles. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. The. Titration Calculations Equation.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Calculations Equation The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Deduce the number of moles. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full. Titration Calculations Equation.
From www.youtube.com
Iron Tablet Titration Calculations Example YouTube Titration Calculations Equation When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. Ma × va = mb × vb. Calculate the amount of sodium hydroxide in moles. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Once a titration is completed. Titration Calculations Equation.
From www.youtube.com
Titration Calculations YouTube Titration Calculations Equation In the naoh—ch 3 cooh reaction eq. Find the number of moles of acid. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Va and vb are the volumes of the acid. Moles of acid = concentration x volume in dm 3. Ma × va. Titration Calculations Equation.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Calculations Equation Calculate the amount of sodium hydroxide in moles. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. How to do titration calculations. Hcl (aq) + naoh (aq) → nacl (aq) + h 2 o. Ma × va = mb × vb. Va and vb are the volumes of the acid.. Titration Calculations Equation.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Calculations Equation Ma is the molarity of the acid, while mb is the molarity of the base. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Calculate. Titration Calculations Equation.
From www.sliderbase.com
Dilution Titration Calculations Equation When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.. Titration Calculations Equation.
From www.numerade.com
SOLVED 'What volume of 0.12M Ba(OH)2 is needed to neutralize 12.2 mL Titration Calculations Equation How to do titration calculations. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. Deduce the number of moles. Calculate the amount of sodium hydroxide in moles. Va and vb are the volumes of the acid. Hcl (aq) + naoh (aq) → nacl (aq) + h 2 o. In the. Titration Calculations Equation.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Calculations Equation Hcl (aq) + naoh (aq) → nacl (aq) + h 2 o. Va and vb are the volumes of the acid. Find the number of moles of acid. Deduce the number of moles. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Moles of acid = concentration x volume. Titration Calculations Equation.
From www.youtube.com
Titration Calculations YouTube Titration Calculations Equation The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Calculate the amount of sodium hydroxide in moles. How to do titration calculations. When hydrochloric acid. Titration Calculations Equation.
From www.scribd.com
Titration Calculations Section A For these questions, your equation Titration Calculations Equation When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. How to do titration calculations. Calculate the amount of sodium hydroxide in moles. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Deduce the number of moles. Hcl (aq). Titration Calculations Equation.
From www.coursehero.com
[Solved] calculate from your titration results the moles of vitamin C Titration Calculations Equation The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Ma × va = mb × vb. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. In the naoh—ch 3 cooh reaction eq. A titration. Titration Calculations Equation.
From www.edplace.com
Understand Advanced Titration Calculations Worksheet EdPlace Titration Calculations Equation In the naoh—ch 3 cooh reaction eq. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. Ma × va = mb × vb. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Calculate the amount of sodium hydroxide in moles. The example below demonstrates the technique. Titration Calculations Equation.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Titration Calculations Equation Find the number of moles of acid. How to do titration calculations. In the naoh—ch 3 cooh reaction eq. When hydrochloric acid is reacted with sodium hydroxide, an acid/base mole ratio of 1:1 is required for full neutralization. Calculate the amount of sodium hydroxide in moles. The object of a titration is always to add just the amount of titrant. Titration Calculations Equation.
From www.youtube.com
Water Hardness (EDTA) Titration Calculations Example YouTube Titration Calculations Equation Ma × va = mb × vb. How to do titration calculations. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Ma is the molarity of the acid, while mb is the molarity of the base. Va and vb are the volumes of the acid. Hcl (aq) +. Titration Calculations Equation.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Calculations Equation Ma is the molarity of the acid, while mb is the molarity of the base. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Find the number of moles of acid. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Deduce the number of moles.. Titration Calculations Equation.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5570905 Titration Calculations Equation The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Ma is the molarity of the acid, while mb is the molarity of the base. Once a titration is completed and the average titre has been calculated, you can. Deduce the number of moles. The example. Titration Calculations Equation.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Calculations Equation Ma is the molarity of the acid, while mb is the molarity of the base. Moles of acid = concentration x volume in dm 3. Ma × va = mb × vb. Calculate the amount of sodium hydroxide in moles. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Deduce the number of moles. The example. Titration Calculations Equation.