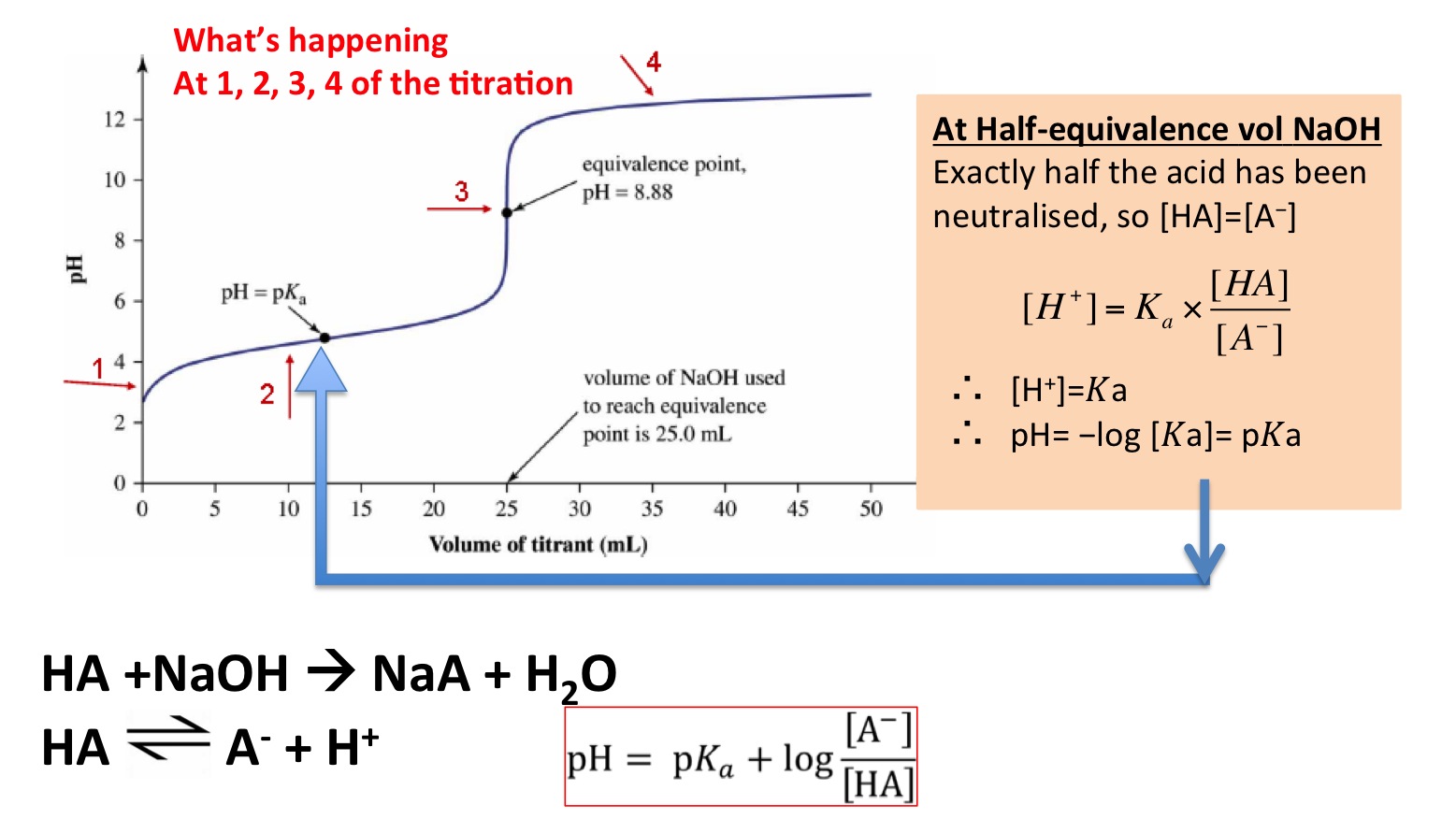
Weak Acid Strong Base Titration Half Equivalence Point . Sorting out some confusing terms. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The equivalence point of a titration. The half that has been titrated has been converted into a. One point in the titration of a weak acid or a weak base is particularly important: Assuming that you're titrating a weak. 0.050 l × 6 mol/l = 0.3 moles of strong acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. The molarity of the acid is given, so the number of moles titrated can be calculated: The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid.
from socratic.org
The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. The molarity of the acid is given, so the number of moles titrated can be calculated: 0.050 l × 6 mol/l = 0.3 moles of strong acid. One point in the titration of a weak acid or a weak base is particularly important: The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. Sorting out some confusing terms. The half that has been titrated has been converted into a. Assuming that you're titrating a weak. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point.
The "pH" at onehalf the equivalence point in an acidbase titration
Weak Acid Strong Base Titration Half Equivalence Point The molarity of the acid is given, so the number of moles titrated can be calculated: The equivalence point of a titration. One point in the titration of a weak acid or a weak base is particularly important: Sorting out some confusing terms. The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. 0.050 l × 6 mol/l = 0.3 moles of strong acid. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The molarity of the acid is given, so the number of moles titrated can be calculated: Assuming that you're titrating a weak. The half that has been titrated has been converted into a.
From www.vrogue.co
How To Calculate Pka From The Half Equivalence Point vrogue.co Weak Acid Strong Base Titration Half Equivalence Point Sorting out some confusing terms. One point in the titration of a weak acid or a weak base is particularly important: The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. 0.050 l × 6 mol/l = 0.3 moles of strong acid. I have learnt. Weak Acid Strong Base Titration Half Equivalence Point.
From erikfersherman.blogspot.com
How to Calculate Ph at Half Equivalence Point Weak Acid Strong Base Titration Half Equivalence Point Sorting out some confusing terms. The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. Assuming that you're titrating a weak. 0.050 l × 6 mol/l = 0.3 moles of strong acid. The half equivalence point is the stage of the titration at which exactly. Weak Acid Strong Base Titration Half Equivalence Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Weak Acid Strong Base Titration Half Equivalence Point The equivalence point of a titration. One point in the titration of a weak acid or a weak base is particularly important: Sorting out some confusing terms. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. The half that has been titrated has been converted into a. The half. Weak Acid Strong Base Titration Half Equivalence Point.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Weak Acid Strong Base Titration Half Equivalence Point The molarity of the acid is given, so the number of moles titrated can be calculated: The half that has been titrated has been converted into a. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. The idea here is that at the half equivalence point, the. Weak Acid Strong Base Titration Half Equivalence Point.
From www.vrogue.co
Acide Base Titration The Half Equivalence Point vrogue.co Weak Acid Strong Base Titration Half Equivalence Point Sorting out some confusing terms. The molarity of the acid is given, so the number of moles titrated can be calculated: 0.050 l × 6 mol/l = 0.3 moles of strong acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. The idea here is that at. Weak Acid Strong Base Titration Half Equivalence Point.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Weak Acid Strong Base Titration Half Equivalence Point 0.050 l × 6 mol/l = 0.3 moles of strong acid. The half that has been titrated has been converted into a. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. Assuming that you're titrating a weak. The equivalence point of a titration. One point in the. Weak Acid Strong Base Titration Half Equivalence Point.
From www.slideshare.net
pH Understanding titration curve Weak Acid Strong Base Titration Half Equivalence Point The equivalence point of a titration. One point in the titration of a weak acid or a weak base is particularly important: The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. 0.050 l × 6 mol/l = 0.3 moles of strong acid. The half. Weak Acid Strong Base Titration Half Equivalence Point.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Weak Acid Strong Base Titration Half Equivalence Point The half that has been titrated has been converted into a. Sorting out some confusing terms. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The molarity of the acid is given, so the number of moles titrated can be calculated: One point in the titration of. Weak Acid Strong Base Titration Half Equivalence Point.
From www.chm.uri.edu
CHM 112 Lecture 19 Weak Acid Strong Base Titration Half Equivalence Point The molarity of the acid is given, so the number of moles titrated can be calculated: Assuming that you're titrating a weak. The half that has been titrated has been converted into a. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. I have learnt that for. Weak Acid Strong Base Titration Half Equivalence Point.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Weak Acid Strong Base Titration Half Equivalence Point The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. The equivalence point of a titration. One point in the titration of a. Weak Acid Strong Base Titration Half Equivalence Point.
From www.vrogue.co
Acide Base Titration The Half Equivalence Point vrogue.co Weak Acid Strong Base Titration Half Equivalence Point The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. One point in the titration of a weak acid or a weak base. Weak Acid Strong Base Titration Half Equivalence Point.
From www.youtube.com
Titration of a weak base with a strong acid Chemistry Khan Academy Weak Acid Strong Base Titration Half Equivalence Point The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. The equivalence point of a titration. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. 0.050 l × 6 mol/l = 0.3 moles of. Weak Acid Strong Base Titration Half Equivalence Point.
From www.youtube.com
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube Weak Acid Strong Base Titration Half Equivalence Point 0.050 l × 6 mol/l = 0.3 moles of strong acid. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The molarity of the acid is given,. Weak Acid Strong Base Titration Half Equivalence Point.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Weak Acid Strong Base Titration Half Equivalence Point The half that has been titrated has been converted into a. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. One point in the titration of a weak acid or a weak base is particularly important: I have learnt that for a weak acid — strong base. Weak Acid Strong Base Titration Half Equivalence Point.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Weak Acid Strong Base Titration Half Equivalence Point The half that has been titrated has been converted into a. Assuming that you're titrating a weak. The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. Sorting out some confusing terms. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a}. Weak Acid Strong Base Titration Half Equivalence Point.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Weak Acid Strong Base Titration Half Equivalence Point The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The half that has been titrated has been converted into a. One point in the titration of a weak acid or a weak base is particularly important: I have learnt that for a weak acid — strong base. Weak Acid Strong Base Titration Half Equivalence Point.
From www.numerade.com
SOLVED When is pKa= pH? equivalence point of a strong acid/strong base Weak Acid Strong Base Titration Half Equivalence Point The equivalence point of a titration. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The half that has been titrated has been converted into a. Sorting out some confusing terms. The titration of a weak acid with a strong base involves the direct transfer of protons. Weak Acid Strong Base Titration Half Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Weak Acid Strong Base Titration Half Equivalence Point Sorting out some confusing terms. One point in the titration of a weak acid or a weak base is particularly important: The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The equivalence point of a titration. The titration of a weak acid with a strong base involves. Weak Acid Strong Base Titration Half Equivalence Point.
From www.chegg.com
Solved Identify the halfequivalence point of the weak Weak Acid Strong Base Titration Half Equivalence Point The half that has been titrated has been converted into a. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. Sorting out some confusing terms. The equivalence point of a titration. The titration of a weak acid with a strong base involves the direct transfer of protons. Weak Acid Strong Base Titration Half Equivalence Point.
From chemistry.stackexchange.com
Shape of WeakStrong AcidBase Titration Chemistry Stack Exchange Weak Acid Strong Base Titration Half Equivalence Point I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. 0.050 l × 6 mol/l = 0.3 moles of strong acid. Assuming that you're titrating a weak. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The. Weak Acid Strong Base Titration Half Equivalence Point.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Weak Acid Strong Base Titration Half Equivalence Point The half that has been titrated has been converted into a. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. One point. Weak Acid Strong Base Titration Half Equivalence Point.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Weak Acid Strong Base Titration Half Equivalence Point Sorting out some confusing terms. The half that has been titrated has been converted into a. The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. 0.050 l × 6 mol/l = 0.3 moles of strong acid. The equivalence point of a titration. The titration. Weak Acid Strong Base Titration Half Equivalence Point.
From www.youtube.com
NCEA L3 Chem Sketching a Weak Acid vs Strong Base Titration Curve Weak Acid Strong Base Titration Half Equivalence Point The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. The equivalence point of a titration. Assuming that you're titrating a weak. Sorting out some confusing terms. The molarity of the acid is given, so the number of moles titrated can be calculated: The idea here is that. Weak Acid Strong Base Titration Half Equivalence Point.
From www.youtube.com
Determining the pH During a Strong AcidWeak Base Titration at Weak Acid Strong Base Titration Half Equivalence Point One point in the titration of a weak acid or a weak base is particularly important: 0.050 l × 6 mol/l = 0.3 moles of strong acid. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. The equivalence point of a titration. The half that has been titrated has. Weak Acid Strong Base Titration Half Equivalence Point.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Weak Acid Strong Base Titration Half Equivalence Point The equivalence point of a titration. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The molarity of the acid is given, so the number of moles titrated can be calculated: The idea here is that at the half equivalence point, the ph of the solution will. Weak Acid Strong Base Titration Half Equivalence Point.
From www.vrogue.co
Acid Base Titration Titration Curves Equivalence Poin vrogue.co Weak Acid Strong Base Titration Half Equivalence Point The equivalence point of a titration. Sorting out some confusing terms. One point in the titration of a weak acid or a weak base is particularly important: The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. The molarity of the acid is given, so the number of. Weak Acid Strong Base Titration Half Equivalence Point.
From studylib.net
Titration Curve weak acid with strong base overshoot Weak Acid Strong Base Titration Half Equivalence Point The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. The half that has been titrated has been converted into a. The equivalence point of a titration. The molarity of the acid is given, so the number of moles titrated can be calculated: 0.050 l × 6 mol/l. Weak Acid Strong Base Titration Half Equivalence Point.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid Weak Acid Strong Base Titration Half Equivalence Point The half that has been titrated has been converted into a. The molarity of the acid is given, so the number of moles titrated can be calculated: I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. 0.050 l × 6 mol/l = 0.3 moles of strong acid. The titration. Weak Acid Strong Base Titration Half Equivalence Point.
From www.reddit.com
Is (half equivalence point) when a conjugate base and an acid are equal Weak Acid Strong Base Titration Half Equivalence Point The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. The half equivalence point is the stage of the titration at which exactly. Weak Acid Strong Base Titration Half Equivalence Point.
From www.youtube.com
Titration Weak base/strong acid Before the equivalence point YouTube Weak Acid Strong Base Titration Half Equivalence Point The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The equivalence point of a titration. One point in the titration of a. Weak Acid Strong Base Titration Half Equivalence Point.
From www.vrogue.co
Acide Base Titration The Half Equivalence Point vrogue.co Weak Acid Strong Base Titration Half Equivalence Point The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. Sorting out some confusing terms. The molarity of the acid is given, so the number of moles titrated can be calculated: 0.050 l × 6 mol/l = 0.3 moles of strong acid. Assuming that you're titrating a weak.. Weak Acid Strong Base Titration Half Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Weak Acid Strong Base Titration Half Equivalence Point The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. One point in the titration of a weak acid or a weak base. Weak Acid Strong Base Titration Half Equivalence Point.
From slideplayer.com
Weak Acid Strong Base Titration ppt download Weak Acid Strong Base Titration Half Equivalence Point 0.050 l × 6 mol/l = 0.3 moles of strong acid. One point in the titration of a weak acid or a weak base is particularly important: I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. The half that has been titrated has been converted into a. The half. Weak Acid Strong Base Titration Half Equivalence Point.
From mungfali.com
Half Equivalence Point On Titration Curve Weak Acid Strong Base Titration Half Equivalence Point The half that has been titrated has been converted into a. The equivalence point of a titration. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. I have learnt that for a weak acid — strong base titration, $\mathrm{p}k_\mathrm{a} = \mathrm{ph}$ at the half equivalence point. Assuming. Weak Acid Strong Base Titration Half Equivalence Point.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Weak Acid Strong Base Titration Half Equivalence Point The idea here is that at the half equivalence point, the ph of the solution will be equal to the pka of the weak acid. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. The molarity of the acid is given, so the number of moles titrated. Weak Acid Strong Base Titration Half Equivalence Point.