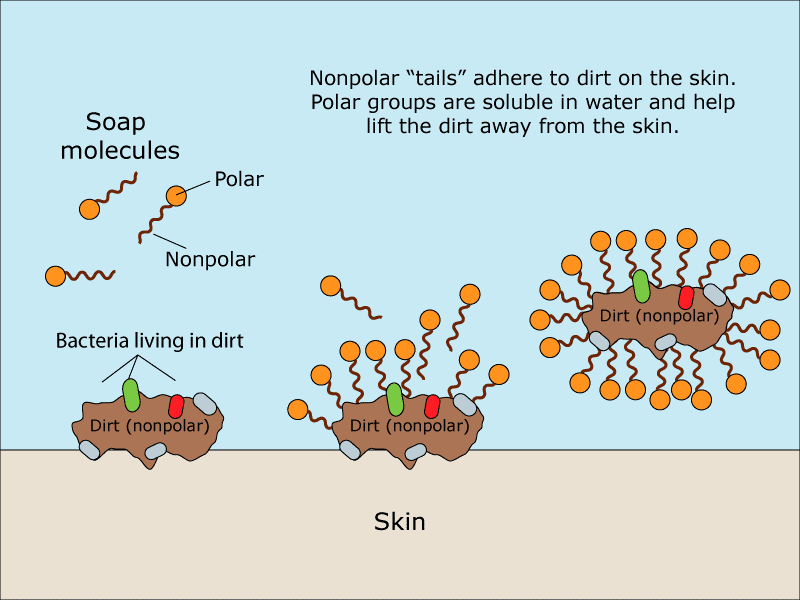
Dish Soap Chemical Structure . Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. This structure explains the cleansing action of soap as the. The structure of soap molecules. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. The hydrophobic tail, derived from. The reaction produces sodium salts of. Soaps and detergents are both surfactants as their. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. But, the most common surfactant in. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Before sodium hydroxide was commercially.
from www.defeatdd.org
Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. But, the most common surfactant in. The reaction produces sodium salts of. Soaps and detergents are both surfactants as their. The structure of soap molecules. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. This structure explains the cleansing action of soap as the. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. Before sodium hydroxide was commercially. The hydrophobic tail, derived from.
How does soap actually work?
Dish Soap Chemical Structure Before sodium hydroxide was commercially. Before sodium hydroxide was commercially. This structure explains the cleansing action of soap as the. The reaction produces sodium salts of. The structure of soap molecules. Soaps and detergents are both surfactants as their. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. But, the most common surfactant in. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived from.
From www.dreamstime.com
General Formula of Solid and Liquid Soap Molecule. RCOONa, RCOOK Dish Soap Chemical Structure The reaction produces sodium salts of. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The structure of soap molecules. But, the most common surfactant in. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soaps and detergents share similar structure as their structures consist. Dish Soap Chemical Structure.
From patents.google.com
WO2014060774A1 Dishwashing detergent composition comprising soapwort Dish Soap Chemical Structure Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. The structure of soap molecules. Before sodium hydroxide was commercially. The reaction produces sodium salts of. This structure. Dish Soap Chemical Structure.
From www.alamy.com
General formula of solid soap molecule. Sodium carboxylate, RCOONa. It Dish Soap Chemical Structure Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The hydrophobic tail, derived from. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The structure of soap. Dish Soap Chemical Structure.
From www.compoundchem.com
The chemistry behind how dishwashers clean Compound Interest Dish Soap Chemical Structure Soaps and detergents are both surfactants as their. The structure of soap molecules. This structure explains the cleansing action of soap as the. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Before sodium hydroxide was commercially. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic. Dish Soap Chemical Structure.
From homeclasp.com
Dish Soap Chemical Formula Dish Soap Chemical Structure Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The structure of soap molecules. The hydrophobic tail, derived from. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. The reaction produces sodium salts of. Before sodium hydroxide was commercially. Soaps and detergents share similar structure. Dish Soap Chemical Structure.
From ar.inspiredpencil.com
Soap Molecule Structure Dish Soap Chemical Structure Before sodium hydroxide was commercially. This structure explains the cleansing action of soap as the. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. But, the most common surfactant in. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived from. Most. Dish Soap Chemical Structure.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Dish Soap Chemical Structure But, the most common surfactant in. The structure of soap molecules. The reaction produces sodium salts of. Before sodium hydroxide was commercially. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. This structure explains the cleansing action of soap as the. Soaps and detergents share similar structure as. Dish Soap Chemical Structure.
From www.writework.com
Soaps and DetergentsBy Nicole Renzi Chemistry 102 Laboratory Section Dish Soap Chemical Structure The reaction produces sodium salts of. Soaps and detergents are both surfactants as their. The structure of soap molecules. This structure explains the cleansing action of soap as the. The hydrophobic tail, derived from. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. But, the most common surfactant in. Soaps and detergents share similar structure as their. Dish Soap Chemical Structure.
From reportd953.web.fc2.com
What's the chemical formula of soap? Dish Soap Chemical Structure Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. The reaction produces sodium salts of. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. The structure of soap molecules. Soap molecules are amphiphilic, meaning they have both hydrophobic and. Dish Soap Chemical Structure.
From aliceinchemiland.blogspot.com
Organic Chemistry in My Daily Life Organic Chemistry about Soap and Dish Soap Chemical Structure Soaps and detergents are both surfactants as their. But, the most common surfactant in. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. The hydrophobic tail, derived from. Before sodium hydroxide was commercially. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and. Dish Soap Chemical Structure.
From homeclasp.com
Dish Soap Chemical Formula Dish Soap Chemical Structure Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The reaction produces sodium salts of. This structure explains the cleansing action of soap as the. The hydrophobic tail, derived from. But, the most common surfactant in.. Dish Soap Chemical Structure.
From dxsqnqafeco.blob.core.windows.net
Home Cleaning Products Formulation at Erich Brunson blog Dish Soap Chemical Structure But, the most common surfactant in. The structure of soap molecules. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. This structure explains the cleansing action of soap as the.. Dish Soap Chemical Structure.
From www.coursehero.com
[Solved] Draw the chemical structure of a soap and a detergent (choose Dish Soap Chemical Structure Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soaps and detergents are both surfactants as their. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soaps and detergents share similar structure as their structures consist. Dish Soap Chemical Structure.
From www.defeatdd.org
How does soap actually work? Dish Soap Chemical Structure The structure of soap molecules. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The reaction produces sodium salts of. This structure explains the cleansing action of soap as the. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. But, the most common surfactant in. Soaps and detergents. Dish Soap Chemical Structure.
From thesoapmoleculeco.com
The Soap Molecule Co. Dish Soap Chemical Structure Before sodium hydroxide was commercially. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived from. The structure of soap molecules. This structure explains the cleansing action of soap as the. Soaps and. Dish Soap Chemical Structure.
From spmchemistry.blog.onlinetuition.com.my
Detergent SPM Chemistry Dish Soap Chemical Structure Soaps and detergents are both surfactants as their. The hydrophobic tail, derived from. But, the most common surfactant in. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. Soaps are cleaning agents that are usually made by reacting alkali. Dish Soap Chemical Structure.
From www.essentialchemicalindustry.org
Surfactants Dish Soap Chemical Structure Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants.. Dish Soap Chemical Structure.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID Dish Soap Chemical Structure This structure explains the cleansing action of soap as the. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived from. Most dishwashing soaps are made up of a chemical formula consisting of. Dish Soap Chemical Structure.
From cosmosmagazine.com
The chemistry of soap Dish Soap Chemical Structure Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. Before sodium hydroxide was commercially. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. This structure explains the cleansing action of soap as the. Most dishwashing soaps are made up of a chemical formula consisting of the soap base. Dish Soap Chemical Structure.
From ar.inspiredpencil.com
Soap Molecule Structure Dish Soap Chemical Structure Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. The reaction produces sodium salts of. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Before sodium hydroxide. Dish Soap Chemical Structure.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Dish Soap Chemical Structure But, the most common surfactant in. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. The hydrophobic tail, derived from. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. This structure explains the cleansing action of soap as the.. Dish Soap Chemical Structure.
From www.slideshare.net
Soap and detergent Dish Soap Chemical Structure Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. But, the most common surfactant in. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. The hydrophobic tail, derived from. The reaction produces sodium salts of. Soap molecules are amphiphilic,. Dish Soap Chemical Structure.
From www.dreamstime.com
General Formula of Solid Soap Molecule. Sodium Carboxylate, RCOONa. it Dish Soap Chemical Structure Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head.. Dish Soap Chemical Structure.
From www.pinterest.com
Pin by Wedding Giveaways on LABELS! LABELS! LABELS! Dishwashing Dish Soap Chemical Structure Soaps and detergents are both surfactants as their. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The structure of soap molecules. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soaps and detergents share similar. Dish Soap Chemical Structure.
From yeserchem.com
Unlocking the Secrets of Dishwashing Liquid Formula A Comprehensive Dish Soap Chemical Structure Soaps and detergents are both surfactants as their. Before sodium hydroxide was commercially. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. But, the most common surfactant in. The structure of soap molecules. This structure explains the cleansing action of soap as the. Soap molecules are amphiphilic, meaning. Dish Soap Chemical Structure.
From courses.lumenlearning.com
17.2 Fats and Oils The Basics of General, Organic, and Biological Dish Soap Chemical Structure The reaction produces sodium salts of. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Before sodium hydroxide was commercially. The structure of soap molecules. But, the most common surfactant in. This structure explains the cleansing action of soap as the. Soaps are cleaning agents that are usually. Dish Soap Chemical Structure.
From ar.inspiredpencil.com
Soap Molecule Structure Dish Soap Chemical Structure Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. The hydrophobic tail, derived from. But, the most common surfactant in. The structure of soap molecules. This structure explains the cleansing action of soap as the. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. Soaps. Dish Soap Chemical Structure.
From ar.inspiredpencil.com
Soap Molecule Structure Dish Soap Chemical Structure Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids.. Dish Soap Chemical Structure.
From www.pinterest.com
Mrs. Meyer's Clean Day 16 Oz. Geranium Scent Liquid Dish Soap Do it Dish Soap Chemical Structure Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. The hydrophobic tail, derived from. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. But, the most common surfactant in. Soaps and detergents are both surfactants as their. The reaction. Dish Soap Chemical Structure.
From www.shutterstock.com
Soap Chemistry Soap Chemistry Formula Structure Stock Vector (Royalty Dish Soap Chemical Structure Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction produces sodium salts of. Before sodium hydroxide was commercially. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soaps and detergents share similar structure as. Dish Soap Chemical Structure.
From www.essentialchemicalindustry.org
Surfactants Dish Soap Chemical Structure Before sodium hydroxide was commercially. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soaps and detergents are both surfactants as their. The hydrophobic tail, derived from. Soaps are cleaning. Dish Soap Chemical Structure.
From www.reddit.com
This dish soap lists the purpose for each ingredient coolguides Dish Soap Chemical Structure The reaction produces sodium salts of. The hydrophobic tail, derived from. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a. Dish Soap Chemical Structure.
From www.pinterest.com
Image result for dish wash soap making formula Soap making Dish Soap Chemical Structure Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. But, the most common surfactant in. Soaps and detergents are both surfactants as their. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap molecules are amphiphilic,. Dish Soap Chemical Structure.
From ar.inspiredpencil.com
Soap Molecule Polar Or Nonpolar Dish Soap Chemical Structure The hydrophobic tail, derived from. The structure of soap molecules. But, the most common surfactant in. Before sodium hydroxide was commercially. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head.. Dish Soap Chemical Structure.
From www.thoughtco.com
How Soap Works Dish Soap Chemical Structure The reaction produces sodium salts of. Soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. Before sodium hydroxide was commercially. The structure of soap molecules. Most dishwashing soaps are made up of a chemical formula consisting of the soap base (c17h35coona) and one or more surfactants. But, the most common surfactant in. Soaps and detergents are both. Dish Soap Chemical Structure.