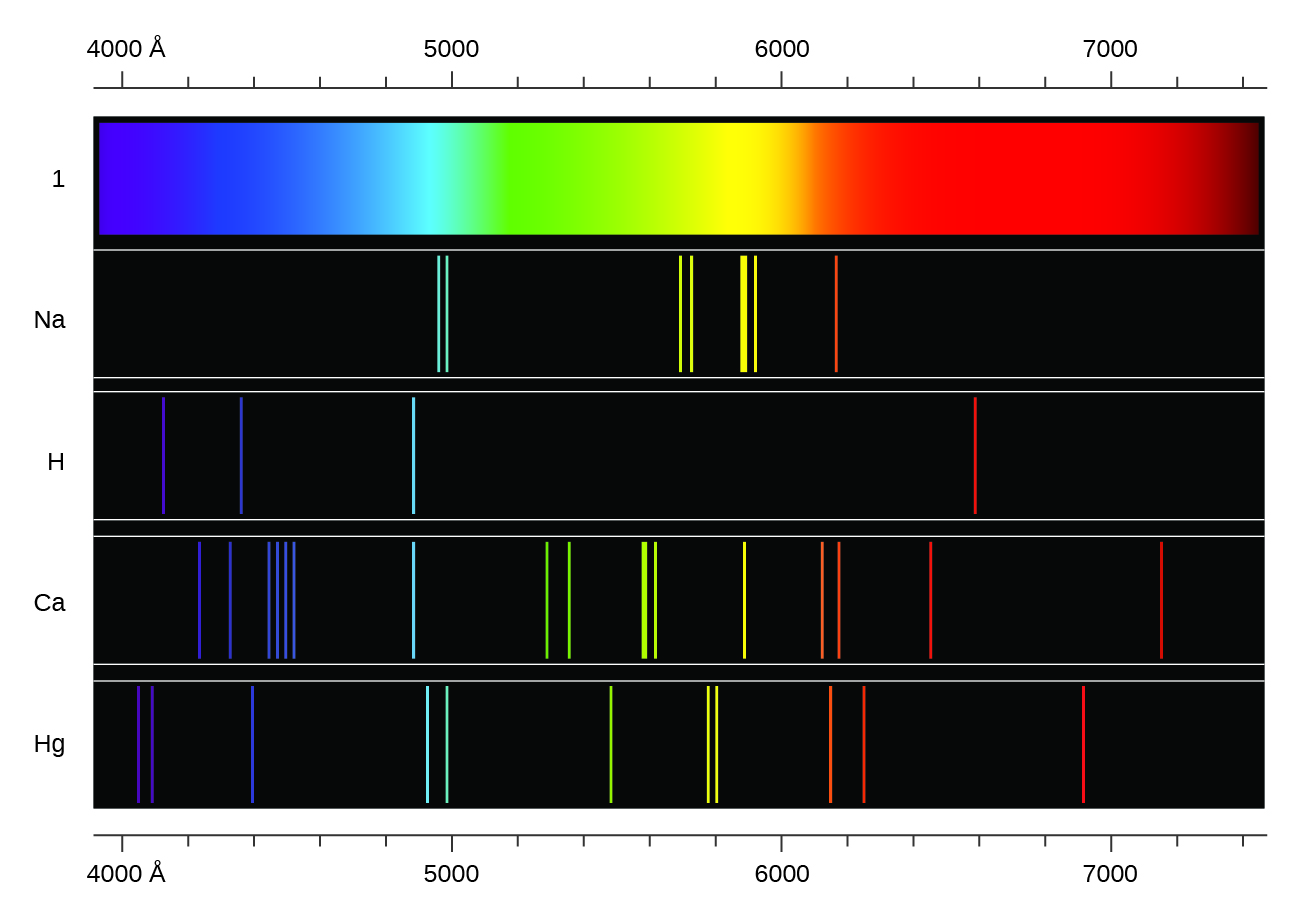
Emission Spectra For Elements . An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. | how can you tell them apart? | don't spectra all look the same? Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Hydrogen (1 electron) helium (2 electrons) carbon (6. If the emission lines of the chemical elements were.
from wisc.pb.unizin.org
Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. If the emission lines of the chemical elements were. | don't spectra all look the same? For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Hydrogen (1 electron) helium (2 electrons) carbon (6. | how can you tell them apart? An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains.
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/
Emission Spectra For Elements | don't spectra all look the same? Hydrogen (1 electron) helium (2 electrons) carbon (6. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. | don't spectra all look the same? | how can you tell them apart? If the emission lines of the chemical elements were.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra For Elements If the emission lines of the chemical elements were. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. Hydrogen (1 electron) helium (2 electrons) carbon (6. An atomic. Emission Spectra For Elements.
From www.sliderbase.com
Emission spectra Presentation Physics Emission Spectra For Elements Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. If the emission lines of the chemical elements were. Hydrogen (1 electron) helium (2 electrons) carbon (6. The actual wavelengths of the lines are predictably. For higher. Emission Spectra For Elements.
From www.reddit.com
Emission Spectra of the Elements in Periodic Table Format r/chemistry Emission Spectra For Elements The actual wavelengths of the lines are predictably. | don't spectra all look the same? The spectroscope separates the light of varying wavelengths and we observe a line spectrum. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. | how can you tell them apart? Atomic emission spectroscopy (aes) is. Emission Spectra For Elements.
From www.pngjoy.com
Mercury Element Emission Spectra Of Elements, Png Download 609x441 Emission Spectra For Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. | how can you tell them apart? | don't spectra all look the same? For higher physics, revise emission or absorption of certain frequencies of light from. Emission Spectra For Elements.
From www.savemyexams.com
Flame Emission Spectroscopy AQA GCSE Chemistry Revision Notes 2018 Emission Spectra For Elements | don't spectra all look the same? Hydrogen (1 electron) helium (2 electrons) carbon (6. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. | how can you tell them apart? An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Emission Spectra For Elements.
From scisyn.com
Spectra Examples Emission Spectra For Elements Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. | how can you tell them apart? The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into. Emission Spectra For Elements.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra For Elements The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. Hydrogen (1 electron) helium (2 electrons) carbon (6. If the emission lines of the chemical elements were. | don't spectra all look the same? For higher physics, revise emission or absorption of certain. Emission Spectra For Elements.
From www.esa.int
ESA Absorption and emission spectra of various elements Emission Spectra For Elements For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. | how can you tell them apart? Hydrogen (1 electron) helium (2 electrons) carbon (6. If the emission lines of the chemical. Emission Spectra For Elements.
From www.resonancescience.org
What is Resonance and Why is it so Important? Emission Spectra For Elements Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of. Emission Spectra For Elements.
From www.pinterest.com
Emission spectra of the elements Teaching chemistry, Science Emission Spectra For Elements For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Hydrogen (1 electron) helium (2 electrons) carbon (6. If the emission lines of the chemical elements were. | how can you tell them apart? An atomic emission spectrum is the pattern of lines formed when light passes through a prism to. Emission Spectra For Elements.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectra For Elements | don't spectra all look the same? Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. If the emission lines of the chemical elements were. Hydrogen (1 electron) helium (2 electrons) carbon (6. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths. Emission Spectra For Elements.
From www.sciencephoto.com
HHeHg emission spectra Stock Image C017/7260 Science Photo Library Emission Spectra For Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. | how can you tell them apart? Hydrogen (1 electron) helium (2 electrons) carbon (6. If the emission lines of the chemical elements were. | don't spectra all look the same? Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental. Emission Spectra For Elements.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra For Elements If the emission lines of the chemical elements were. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. | how can you tell them apart? An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light. Emission Spectra For Elements.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectra For Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Hydrogen (1 electron) helium (2 electrons) carbon (6. | don't spectra all look the same? An atomic emission spectrum is the pattern of lines formed when light. Emission Spectra For Elements.
From mrsmorrittscience.weebly.com
Emission Spectra of the Elements Arranged on the Periodic Table Mrs Emission Spectra For Elements | don't spectra all look the same? An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Hydrogen (1 electron) helium. Emission Spectra For Elements.
From mungfali.com
Atomic Emission Spectrum Of Elements Emission Spectra For Elements | how can you tell them apart? The spectroscope separates the light of varying wavelengths and we observe a line spectrum. If the emission lines of the chemical elements were. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. | don't spectra all look the same? For higher physics, revise. Emission Spectra For Elements.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra For Elements Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. If the emission lines of the chemical elements were. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Hydrogen (1 electron) helium (2 electrons) carbon (6. | how can you tell. Emission Spectra For Elements.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Emission Spectra For Elements Hydrogen (1 electron) helium (2 electrons) carbon (6. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An atomic emission spectrum is the pattern of lines formed when light passes through. Emission Spectra For Elements.
From mavink.com
Line Emission Spectra Of Elements Emission Spectra For Elements Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Hydrogen (1 electron) helium (2 electrons) carbon (6. The actual wavelengths of the lines. Emission Spectra For Elements.
From www.alamy.com
HHeHg emission spectra. Graphical representation of the emission Emission Spectra For Elements | don't spectra all look the same? The spectroscope separates the light of varying wavelengths and we observe a line spectrum. If the emission lines of the chemical elements were. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An atomic emission spectrum is the pattern of lines formed when. Emission Spectra For Elements.
From umop.net
Visible Spectra of the Elements Emission Spectra For Elements Hydrogen (1 electron) helium (2 electrons) carbon (6. If the emission lines of the chemical elements were. | don't spectra all look the same? | how can you tell them apart? The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. For higher physics, revise emission or absorption. Emission Spectra For Elements.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra For Elements | don't spectra all look the same? An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. | how can you. Emission Spectra For Elements.
From umop.net
Visible Spectra of the Elements Emission Spectra For Elements The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. | how can you tell them apart? An atomic emission spectrum is the pattern of lines formed when light. Emission Spectra For Elements.
From universetoday.com
Spectroscopy The Key to Humanity’s Future in Space Emission Spectra For Elements An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. For higher physics, revise emission or. Emission Spectra For Elements.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra For Elements | don't spectra all look the same? If the emission lines of the chemical elements were. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used. Emission Spectra For Elements.
From www.alamy.com
Diagram showing the absorption and emission spectra of the elements Emission Spectra For Elements Hydrogen (1 electron) helium (2 electrons) carbon (6. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The actual wavelengths of the lines are predictably. If the emission lines of the chemical elements were. | how. Emission Spectra For Elements.
From studylib.net
Every element has its own characteristic spectral emission Emission Spectra For Elements | how can you tell them apart? Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. For higher physics, revise emission or absorption. Emission Spectra For Elements.
From www.carolina.com
Periodic Table of Spectra, Poster Carolina Biological Supply Emission Spectra For Elements Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. | how can you tell them apart? The actual wavelengths of the lines are predictably. | don't spectra all look the same? The spectroscope separates the light of varying wavelengths and we observe a line spectrum. If the emission lines of. Emission Spectra For Elements.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Emission Spectra For Elements Hydrogen (1 electron) helium (2 electrons) carbon (6. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. | don't spectra all look the same? The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we. Emission Spectra For Elements.
From www.reddit.com
Periodic table of each elements emission spectrum with regular periodic Emission Spectra For Elements The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. | don't spectra all look the same? If the emission lines of the chemical elements were. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different. Emission Spectra For Elements.
From www.astronoo.com
Spectroscopie — Astronoo Emission Spectra For Elements | don't spectra all look the same? For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are. Emission Spectra For Elements.
From studylib.net
Emission Spectra of Elements Emission Spectra For Elements If the emission lines of the chemical elements were. | how can you tell them apart? The spectroscope separates the light of varying wavelengths and we observe a line spectrum. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. An atomic emission spectrum is the pattern of lines formed when. Emission Spectra For Elements.
From uwaterloo.ca
Periodic Table of emission spectra Chem 13 News Magazine University Emission Spectra For Elements | don't spectra all look the same? For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Hydrogen (1 electron) helium (2 electrons) carbon. Emission Spectra For Elements.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra For Elements Hydrogen (1 electron) helium (2 electrons) carbon (6. If the emission lines of the chemical elements were. | how can you tell them apart? For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission. Emission Spectra For Elements.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra For Elements Hydrogen (1 electron) helium (2 electrons) carbon (6. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. | don't spectra all look the same? The spectroscope separates the light of varying wavelengths and we observe a line spectrum. For higher physics, revise emission or absorption of certain frequencies of light. Emission Spectra For Elements.