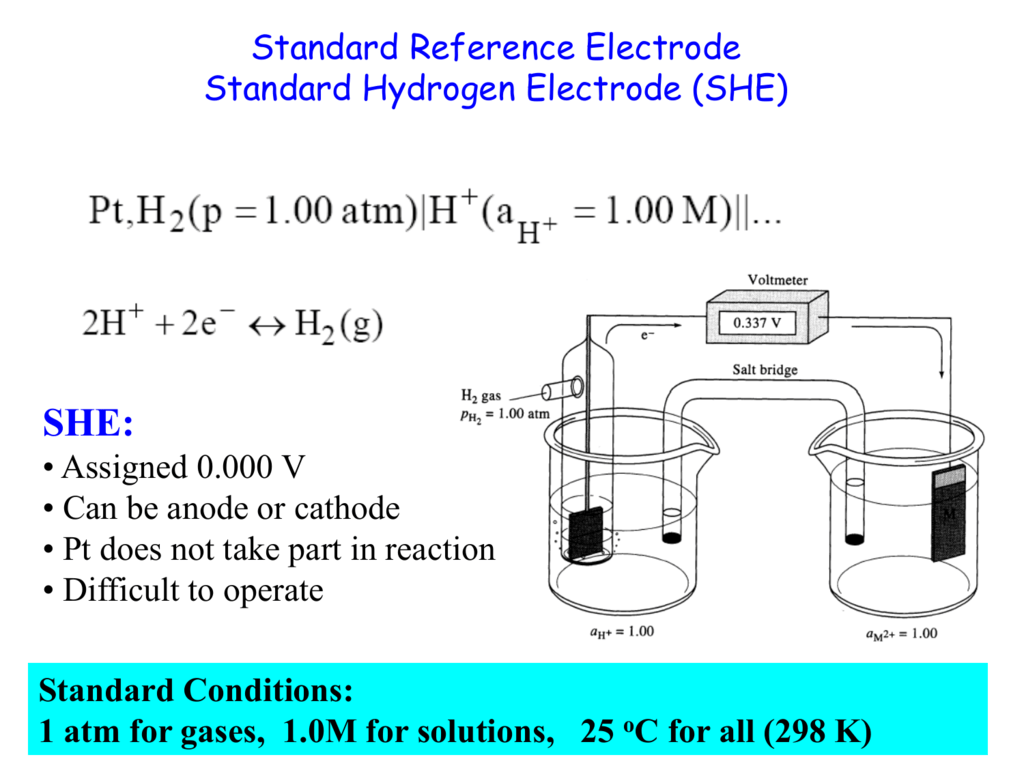
Glass Electrode Cell Representation . In potentiometry we measure the potential of an electrochemical cell under static conditions. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. Specifically for silver/silver chloride electrodes. If both species in a half reaction are aqueous then an inert platinum. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. Because no current—or only a negligible current—flows through the electrochemical cell, its. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. It provides information about the activity of hydronium ions, h3o+, in water.
from dxoqyggvw.blob.core.windows.net
The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. Specifically for silver/silver chloride electrodes. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. Because no current—or only a negligible current—flows through the electrochemical cell, its. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. If both species in a half reaction are aqueous then an inert platinum. In potentiometry we measure the potential of an electrochemical cell under static conditions. It provides information about the activity of hydronium ions, h3o+, in water.
Electrode Nomenclature Meaning at Paul Jones blog
Glass Electrode Cell Representation A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. In potentiometry we measure the potential of an electrochemical cell under static conditions. Specifically for silver/silver chloride electrodes. If both species in a half reaction are aqueous then an inert platinum. Because no current—or only a negligible current—flows through the electrochemical cell, its. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. It provides information about the activity of hydronium ions, h3o+, in water. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used.
From www.researchgate.net
Scheme of a classical threeelectrode cell. Download Scientific Diagram Glass Electrode Cell Representation In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. Specifically for silver/silver chloride electrodes. If both species in a half reaction are aqueous then an inert platinum. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely. Glass Electrode Cell Representation.
From mungfali.com
Glass Electrode Diagram Glass Electrode Cell Representation In potentiometry we measure the potential of an electrochemical cell under static conditions. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. It provides information about the activity of hydronium ions, h3o+, in water. Because no current—or only a negligible current—flows through the electrochemical cell, its. Specifically for silver/silver. Glass Electrode Cell Representation.
From www.researchgate.net
Schematic of a glass cell setup with rotating cylinder electrode Glass Electrode Cell Representation If both species in a half reaction are aqueous then an inert platinum. In potentiometry we measure the potential of an electrochemical cell under static conditions. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation. Glass Electrode Cell Representation.
From mungfali.com
Glass Electrode Diagram Glass Electrode Cell Representation A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. In potentiometry we measure the potential of an electrochemical cell under static conditions. If both species in a half reaction are aqueous then an inert platinum. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation. Glass Electrode Cell Representation.
From www.researchgate.net
Schematic representation of three electrode electrochemical cell used Glass Electrode Cell Representation This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. Because no current—or only a negligible current—flows through the electrochemical cell, its. A comprehensive article covering how. Glass Electrode Cell Representation.
From derangedphysiology.com
History of the glass electrode Deranged Physiology Glass Electrode Cell Representation A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. If both species in a half reaction are aqueous then an inert platinum. Specifically for silver/silver chloride electrodes. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. In general, if a galvanic cell is connected. Glass Electrode Cell Representation.
From www.protocols.io
Calibration of glass electrode halfcells Glass Electrode Cell Representation If both species in a half reaction are aqueous then an inert platinum. In potentiometry we measure the potential of an electrochemical cell under static conditions. Specifically for silver/silver chloride electrodes. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. It provides information about the activity of hydronium ions, h3o+, in water. The construction of. Glass Electrode Cell Representation.
From www.priyamstudycentre.com
Glass Electrode pH Measurement Glass Electrode Cell Representation Because no current—or only a negligible current—flows through the electrochemical cell, its. In potentiometry we measure the potential of an electrochemical cell under static conditions. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. This method of representing electrochemical cells is known as the conventional representation. Glass Electrode Cell Representation.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Glass Electrode Cell Representation Because no current—or only a negligible current—flows through the electrochemical cell, its. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. In potentiometry we measure the potential of an electrochemical cell under static conditions. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter. Glass Electrode Cell Representation.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom Glass Electrode Cell Representation Specifically for silver/silver chloride electrodes. Because no current—or only a negligible current—flows through the electrochemical cell, its. In potentiometry we measure the potential of an electrochemical cell under static conditions. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. If both species in a half reaction. Glass Electrode Cell Representation.
From www.researchgate.net
Schematic illustration of a fourelectrode glass cell with the RRDE Glass Electrode Cell Representation Specifically for silver/silver chloride electrodes. It provides information about the activity of hydronium ions, h3o+, in water. In potentiometry we measure the potential of an electrochemical cell under static conditions. If both species in a half reaction are aqueous then an inert platinum. Because no current—or only a negligible current—flows through the electrochemical cell, its. The construction of a typical. Glass Electrode Cell Representation.
From www.slideserve.com
PPT ION SELECTIVE ELECTRODES PowerPoint Presentation, free download Glass Electrode Cell Representation This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. In potentiometry we measure the potential of an electrochemical cell under static conditions. It provides information about the activity of hydronium ions, h3o+, in water. Because no current—or only a negligible current—flows through the electrochemical cell, its. In general, if. Glass Electrode Cell Representation.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration Glass Electrode Cell Representation In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. It provides information about the activity of hydronium ions, h3o+, in water. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. In potentiometry. Glass Electrode Cell Representation.
From www.wikilectures.eu
Glass Electrode / Details WikiLectures Glass Electrode Cell Representation Because no current—or only a negligible current—flows through the electrochemical cell, its. It provides information about the activity of hydronium ions, h3o+, in water. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. If both species in a half reaction are aqueous then an inert platinum.. Glass Electrode Cell Representation.
From www.researchgate.net
5 (a) Glass electrode (b) Combined electrode Download Scientific Diagram Glass Electrode Cell Representation This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. It provides information about the activity of hydronium ions, h3o+, in water. Specifically for silver/silver chloride electrodes. Because no current—or only a negligible current—flows through the electrochemical cell, its. In general, if a galvanic cell is connected to a voltmeter,. Glass Electrode Cell Representation.
From dxoqyggvw.blob.core.windows.net
Electrode Nomenclature Meaning at Paul Jones blog Glass Electrode Cell Representation This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. If both species in a half reaction are aqueous then an inert platinum. In potentiometry we measure the potential of an electrochemical cell under static conditions. It provides information about the activity of hydronium ions, h3o+, in water. A comprehensive. Glass Electrode Cell Representation.
From mungfali.com
Glass Electrode Diagram Glass Electrode Cell Representation A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. If both species in a half reaction are aqueous then an inert platinum. In potentiometry we measure the potential of an electrochemical cell under static conditions. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation. Glass Electrode Cell Representation.
From www.researchgate.net
(PDF) Recent Advances in Optical, Electrochemical, and Field Effect pH Glass Electrode Cell Representation It provides information about the activity of hydronium ions, h3o+, in water. If both species in a half reaction are aqueous then an inert platinum. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. A comprehensive article covering how glass electrodes measure ph in a simple,. Glass Electrode Cell Representation.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Glass Electrode Cell Representation The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. If both species in a half reaction are aqueous then an inert platinum. It provides information about the activity of hydronium ions, h3o+, in water. This method of representing electrochemical cells is known as the conventional representation. Glass Electrode Cell Representation.
From www.youtube.com
Write a note on Glass electrode. Electrochemistry Physical Chemistry Glass Electrode Cell Representation It provides information about the activity of hydronium ions, h3o+, in water. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. If both species in a half reaction are aqueous then an. Glass Electrode Cell Representation.
From www.slideserve.com
PPT 3.1 Ion selective Potentiometry PowerPoint Presentation, free Glass Electrode Cell Representation The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. It provides information about the activity of hydronium ions, h3o+, in water. Because no. Glass Electrode Cell Representation.
From www.researchgate.net
Voltage output using glass based electrode. Download Scientific Diagram Glass Electrode Cell Representation Because no current—or only a negligible current—flows through the electrochemical cell, its. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. It provides information about the activity of hydronium ions, h3o+, in water. In general, if a galvanic cell is connected to a voltmeter, the electrode. Glass Electrode Cell Representation.
From mungfali.com
Glass Electrode Diagram Glass Electrode Cell Representation In potentiometry we measure the potential of an electrochemical cell under static conditions. If both species in a half reaction are aqueous then an inert platinum. Specifically for silver/silver chloride electrodes. It provides information about the activity of hydronium ions, h3o+, in water. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16,. Glass Electrode Cell Representation.
From www.researchgate.net
Schematic diagram of threeelectrode cell assembly Download Glass Electrode Cell Representation This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. Because no current—or only a negligible current—flows through the electrochemical cell, its. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. In potentiometry we measure the potential of an electrochemical cell under static conditions. It. Glass Electrode Cell Representation.
From corrtest.en.made-in-china.com
China Glass Electrolytic Cell China Electrochemical Cell, Electrode Glass Electrode Cell Representation A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. In potentiometry we measure the potential of an electrochemical cell under static conditions. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. The construction of a typical electrochemical cell based on a glass electrode is. Glass Electrode Cell Representation.
From www.youtube.com
CHEM2 Component of glass electrode YouTube Glass Electrode Cell Representation This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. It provides information about the activity of hydronium ions, h3o+, in water. If both species in a half reaction are aqueous then an inert platinum. The construction of a typical electrochemical cell based on a glass electrode is shown in. Glass Electrode Cell Representation.
From www.researchgate.net
Schematic representation of three electrode electrochemical cell used Glass Electrode Cell Representation In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. In potentiometry we measure the potential of an electrochemical cell under static conditions. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. If. Glass Electrode Cell Representation.
From www.researchgate.net
Schematic diagram of the glass cell and electrodes used for Glass Electrode Cell Representation Because no current—or only a negligible current—flows through the electrochemical cell, its. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. Specifically for silver/silver chloride electrodes. It provides information about the activity of hydronium ions, h3o+, in water. A comprehensive article covering how glass electrodes measure. Glass Electrode Cell Representation.
From gamma.app
Glass Electrode Glass Electrode Cell Representation This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. If both species in a half reaction are aqueous then an inert platinum. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. In potentiometry we measure. Glass Electrode Cell Representation.
From semcouniversity.com
How the three electrode system works Semco University Semco Glass Electrode Cell Representation This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. Specifically for silver/silver chloride electrodes. A comprehensive article covering how glass electrodes measure ph in a simple,. Glass Electrode Cell Representation.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Glass Electrode Cell Representation It provides information about the activity of hydronium ions, h3o+, in water. Specifically for silver/silver chloride electrodes. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. Because no current—or only a negligible current—flows through the electrochemical cell, its. The construction of a typical electrochemical cell based. Glass Electrode Cell Representation.
From www.youtube.com
Glass Electrode I Indicator Electrode YouTube Glass Electrode Cell Representation The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including a representation of the physical. In general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. In potentiometry we measure the potential of an electrochemical cell under static conditions. Specifically. Glass Electrode Cell Representation.
From www.researchgate.net
(a) Schematic representation of the twoelectrodecell method and (b Glass Electrode Cell Representation In potentiometry we measure the potential of an electrochemical cell under static conditions. It provides information about the activity of hydronium ions, h3o+, in water. Because no current—or only a negligible current—flows through the electrochemical cell, its. Specifically for silver/silver chloride electrodes. The construction of a typical electrochemical cell based on a glass electrode is shown in figure 16, including. Glass Electrode Cell Representation.
From www.researchgate.net
Schematics of threeelectrode cell, standard calomel electrode as Glass Electrode Cell Representation Because no current—or only a negligible current—flows through the electrochemical cell, its. Specifically for silver/silver chloride electrodes. If both species in a half reaction are aqueous then an inert platinum. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. The construction of a typical electrochemical cell based on a. Glass Electrode Cell Representation.
From glossary.periodni.com
Chemistry Glossary Search results for 'electrode of the first kind' Glass Electrode Cell Representation In potentiometry we measure the potential of an electrochemical cell under static conditions. This method of representing electrochemical cells is known as the conventional representation of a cell, and it is widely used. Specifically for silver/silver chloride electrodes. A comprehensive article covering how glass electrodes measure ph in a simple, understandable format. The construction of a typical electrochemical cell based. Glass Electrode Cell Representation.