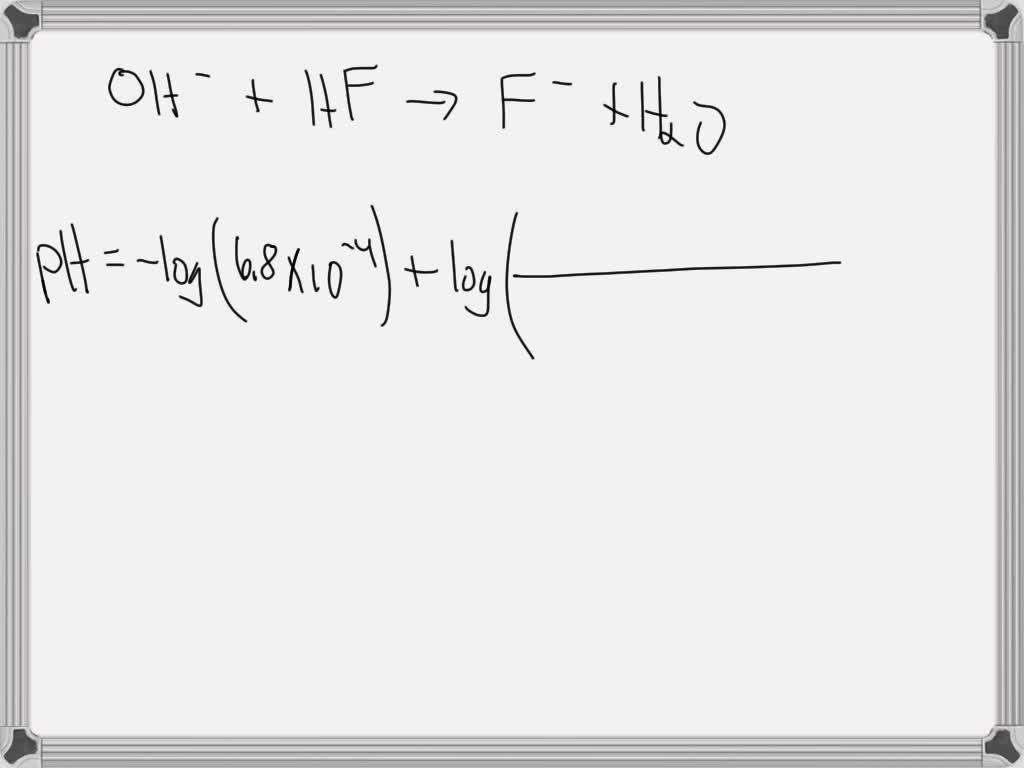Koh H2So4 Titration . You can reduce the cognitive load by careful scaffolding using the table method. Our titration calculator will help you never have to ask how do i calculate titrations? again. A student titrated a 25.0 cm3. Next, pour about 5ml o. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. Titration of sulfuric acid and sodium hydroxide. A titration’s end point was determined using. Record the identification letter of the h2so4. Acid from the acid beaker into the rinse beaker.
from www.numerade.com
You can reduce the cognitive load by careful scaffolding using the table method. Record the identification letter of the h2so4. A student titrated a 25.0 cm3. A titration’s end point was determined using. Titration of sulfuric acid and sodium hydroxide. Our titration calculator will help you never have to ask how do i calculate titrations? again. Acid from the acid beaker into the rinse beaker. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Next, pour about 5ml o. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known.
Determine the pH during the titration of 30 mL of 3.0M KOH with 1.5M
Koh H2So4 Titration Acid from the acid beaker into the rinse beaker. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Our titration calculator will help you never have to ask how do i calculate titrations? again. Acid from the acid beaker into the rinse beaker. Record the identification letter of the h2so4. A titration’s end point was determined using. Titration of sulfuric acid and sodium hydroxide. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. You can reduce the cognitive load by careful scaffolding using the table method. A student titrated a 25.0 cm3. Next, pour about 5ml o.
From www.numerade.com
SOLVED 'The reaction between potassium hydroxide and sulfuric acid is Koh H2So4 Titration Record the identification letter of the h2so4. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. A titration’s end point was determined using. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration. Koh H2So4 Titration.
From www.youtube.com
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Koh H2So4 Titration You can reduce the cognitive load by careful scaffolding using the table method. A student titrated a 25.0 cm3. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Acid from the acid beaker into the rinse beaker. Next, pour about 5ml o. Our titration calculator will help you. Koh H2So4 Titration.
From mavink.com
H2so4 Titration Curve Koh H2So4 Titration One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. Titration of sulfuric acid and sodium hydroxide. Our titration calculator will help you never have to ask how do i calculate titrations? again. You can reduce the cognitive. Koh H2So4 Titration.
From www.youtube.com
KOH+H2SO4=K2SO4+H2O Balance the chemical equation mydocumentary838 Koh H2So4 Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. Record the identification letter of the h2so4. A titration’s end point was determined using. A student titrated a 25.0 cm3. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Acid from the. Koh H2So4 Titration.
From sydney-bogspotmueller.blogspot.com
Potassium Hydroxide and Sulphuric Acid Koh H2So4 Titration Acid from the acid beaker into the rinse beaker. You can reduce the cognitive load by careful scaffolding using the table method. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration’s end point was determined using. Record the identification letter of the h2so4. One of the most basic experiments taught to. Koh H2So4 Titration.
From mavink.com
Titration Labeled Koh H2So4 Titration A titration’s end point was determined using. Titration of sulfuric acid and sodium hydroxide. A student titrated a 25.0 cm3. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Record the identification letter of the h2so4. One of the most basic experiments taught to everyone in school, the. Koh H2So4 Titration.
From www.chegg.com
Solved H2SO4+2NaOH 2H2O+Na2SO4 (12) What volume of a Koh H2So4 Titration A titration’s end point was determined using. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. Record the identification letter of the h2so4. Our titration calculator will help you never have to ask how do i calculate. Koh H2So4 Titration.
From www.coursehero.com
[Solved] A 25.00 ml volume of sulfuric acid was titrated with a Koh H2So4 Titration A student titrated a 25.0 cm3. Next, pour about 5ml o. A titration’s end point was determined using. Titration of sulfuric acid and sodium hydroxide. Record the identification letter of the h2so4. Acid from the acid beaker into the rinse beaker. One of the most basic experiments taught to everyone in school, the titration between an acid and a base. Koh H2So4 Titration.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID2373886 Koh H2So4 Titration Titration of sulfuric acid and sodium hydroxide. A titration’s end point was determined using. Next, pour about 5ml o. A student titrated a 25.0 cm3. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Our titration calculator will help you never have to ask how do i calculate. Koh H2So4 Titration.
From www.numerade.com
Determine the pH during the titration of 30 mL of 3.0M KOH with 1.5M Koh H2So4 Titration You can reduce the cognitive load by careful scaffolding using the table method. Record the identification letter of the h2so4. Next, pour about 5ml o. Our titration calculator will help you never have to ask how do i calculate titrations? again. A student titrated a 25.0 cm3. Acid from the acid beaker into the rinse beaker. In a titration of. Koh H2So4 Titration.
From www.facebook.com
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Koh H2So4 Titration Titration of sulfuric acid and sodium hydroxide. A student titrated a 25.0 cm3. Record the identification letter of the h2so4. A titration’s end point was determined using. Acid from the acid beaker into the rinse beaker. Our titration calculator will help you never have to ask how do i calculate titrations? again. Next, pour about 5ml o. You can reduce. Koh H2So4 Titration.
From www.chegg.com
Solved A student carried out a titration using H2SO4 and Koh H2So4 Titration Next, pour about 5ml o. Our titration calculator will help you never have to ask how do i calculate titrations? again. A student titrated a 25.0 cm3. A titration’s end point was determined using. Titration of sulfuric acid and sodium hydroxide. Record the identification letter of the h2so4. You can reduce the cognitive load by careful scaffolding using the table. Koh H2So4 Titration.
From www.chemielounge.de
Titration Schwefelsäure Berechnung der pHWerte der Äquivalenzpunkte Koh H2So4 Titration Next, pour about 5ml o. A titration’s end point was determined using. Record the identification letter of the h2so4. Titration of sulfuric acid and sodium hydroxide. A student titrated a 25.0 cm3. Acid from the acid beaker into the rinse beaker. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h. Koh H2So4 Titration.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Koh H2So4 Titration Titration of sulfuric acid and sodium hydroxide. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. A student titrated a 25.0 cm3. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to. Koh H2So4 Titration.
From www.youtube.com
KMnO4+H2SO4=K2SO4+MnSO4+H2O+O2 balance the chemical equation Koh H2So4 Titration Acid from the acid beaker into the rinse beaker. Next, pour about 5ml o. A student titrated a 25.0 cm3. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. Our titration calculator will help you never have. Koh H2So4 Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Koh H2So4 Titration One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. You can reduce the cognitive load by careful scaffolding using the table method. Record the identification letter of the h2so4. Next, pour about 5ml o. A student titrated. Koh H2So4 Titration.
From www.numerade.com
SOLVED Describe what happens at the molecular level when the Koh H2So4 Titration You can reduce the cognitive load by careful scaffolding using the table method. A student titrated a 25.0 cm3. A titration’s end point was determined using. Acid from the acid beaker into the rinse beaker. Titration of sulfuric acid and sodium hydroxide. Our titration calculator will help you never have to ask how do i calculate titrations? again. In a. Koh H2So4 Titration.
From www.chegg.com
Solved (a) A titration is performed to determine the Koh H2So4 Titration Next, pour about 5ml o. A titration’s end point was determined using. A student titrated a 25.0 cm3. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Acid from the acid beaker into the rinse beaker. One of the most basic experiments taught to everyone in school, the. Koh H2So4 Titration.
From www.youtube.com
How to Write the Net Ionic Equation for KOH + H2SO4 = K2SO4 + H2O YouTube Koh H2So4 Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. You can reduce the cognitive load by careful scaffolding using the table method. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. One of the most basic experiments taught to everyone in. Koh H2So4 Titration.
From www.numerade.com
Based on the titration curve shown below, what's the pKa of the acid Koh H2So4 Titration In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. Titration of sulfuric acid and sodium hydroxide.. Koh H2So4 Titration.
From www.numerade.com
A student carried out a titration using H2SO4 and KOH. The balanced Koh H2So4 Titration Titration of sulfuric acid and sodium hydroxide. A student titrated a 25.0 cm3. Next, pour about 5ml o. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. Acid from the acid beaker into the rinse beaker. You. Koh H2So4 Titration.
From studylib.net
AcidBase Titration Answers, Sources of Error Koh H2So4 Titration A student titrated a 25.0 cm3. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. Acid from the acid beaker into the rinse beaker. Next, pour about 5ml o. Our titration calculator will help you never have. Koh H2So4 Titration.
From byjus.com
calculate the PH of solution containing 0.98g H2SO4 in 100 mL of water Koh H2So4 Titration In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Next, pour about 5ml o. A titration’s end point was determined using. Acid from the acid beaker into the rinse beaker. You can reduce the cognitive load by careful scaffolding using the table method. Our titration calculator will help. Koh H2So4 Titration.
From www.numerade.com
SOLVED During a titration, 10.00 mL of sulphuric acid, H2SO4 is Koh H2So4 Titration You can reduce the cognitive load by careful scaffolding using the table method. Acid from the acid beaker into the rinse beaker. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Record the identification letter of the h2so4. A titration’s end point was determined using. One of the. Koh H2So4 Titration.
From giasudiem10.edu.vn
KOH + H2SO4 Phương trình hóa học và bài tập vận dụng Koh H2So4 Titration Record the identification letter of the h2so4. Next, pour about 5ml o. Acid from the acid beaker into the rinse beaker. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. A titration’s end point was determined using.. Koh H2So4 Titration.
From www.assignmentexpert.com
Answer in General Chemistry for cath 350286 Koh H2So4 Titration Titration of sulfuric acid and sodium hydroxide. Next, pour about 5ml o. A titration’s end point was determined using. A student titrated a 25.0 cm3. Our titration calculator will help you never have to ask how do i calculate titrations? again. You can reduce the cognitive load by careful scaffolding using the table method. In a titration of sulfuric acid. Koh H2So4 Titration.
From znanija.com
2KOH + H2SO4 = K2SO4 + 2H2O Помогите составить ионное и сокращённое Koh H2So4 Titration Acid from the acid beaker into the rinse beaker. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Next, pour about 5ml o. A titration’s end point was determined using. One of the most basic experiments taught to everyone in school, the titration between an acid and a. Koh H2So4 Titration.
From www.azom.com
Titrating Acid Mixtures Koh H2So4 Titration A titration’s end point was determined using. Record the identification letter of the h2so4. You can reduce the cognitive load by careful scaffolding using the table method. A student titrated a 25.0 cm3. Next, pour about 5ml o. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to. Koh H2So4 Titration.
From slideplayer.com
Acid Base Titrations Lesson ppt download Koh H2So4 Titration Record the identification letter of the h2so4. You can reduce the cognitive load by careful scaffolding using the table method. Next, pour about 5ml o. A titration’s end point was determined using. Acid from the acid beaker into the rinse beaker. One of the most basic experiments taught to everyone in school, the titration between an acid and a base. Koh H2So4 Titration.
From brainly.in
100 mL water sample requires 4 mL 0.02 N H2SO4 for neutralization upto Koh H2So4 Titration A student titrated a 25.0 cm3. Titration of sulfuric acid and sodium hydroxide. You can reduce the cognitive load by careful scaffolding using the table method. Our titration calculator will help you never have to ask how do i calculate titrations? again. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of. Koh H2So4 Titration.
From www.chegg.com
Solved 4. Consider the titration of 50.0 ml of 2.0 KOH. Koh H2So4 Titration Next, pour about 5ml o. Acid from the acid beaker into the rinse beaker. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Record the identification letter of the h2so4. You can reduce the cognitive load by careful scaffolding using the table method. A student titrated a 25.0. Koh H2So4 Titration.
From www.chegg.com
Solved Write the balanced neutralization reaction between Koh H2So4 Titration Titration of sulfuric acid and sodium hydroxide. Record the identification letter of the h2so4. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. Our titration calculator will help you never have to ask how do i calculate. Koh H2So4 Titration.
From www.studypool.com
SOLUTION Solution for a student carried out a titration using h2so4 Koh H2So4 Titration In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. A student titrated a 25.0 cm3. Record the identification letter of the h2so4. One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of. Koh H2So4 Titration.
From mungfali.com
The Titration Of 25 0 Ml Of An Unknown Concentration Of H2so4 Solution 699 Koh H2So4 Titration You can reduce the cognitive load by careful scaffolding using the table method. Next, pour about 5ml o. A titration’s end point was determined using. Our titration calculator will help you never have to ask how do i calculate titrations? again. Titration of sulfuric acid and sodium hydroxide. Acid from the acid beaker into the rinse beaker. In a titration. Koh H2So4 Titration.
From www.numerade.com
SOLVED Using the following data of the results of a titration with Koh H2So4 Titration Acid from the acid beaker into the rinse beaker. You can reduce the cognitive load by careful scaffolding using the table method. Titration of sulfuric acid and sodium hydroxide. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Our titration calculator will help you never have to ask. Koh H2So4 Titration.