Dilute Solution Of Ethanoic Acid . All ethanoic acids have different. when solutions are described as dilute or concentrated: there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. By contrast, strong acids like hydrochloric. when solutions are described as dilute or concentrated: The majority of ethanoic acid is now. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. A dilute solution contains a relatively small amount of dissolved. ethanoic acid (ch 3 cooh): It is the 2nd member of a homologous series of carboxylic acid. A dilute solution contains a relatively small amount of. It therefore has a high dissociation. It is also known as acetic acid.
from www.chegg.com
ethanoic acid (ch 3 cooh): It therefore has a high dissociation. when solutions are described as dilute or concentrated: A dilute solution contains a relatively small amount of dissolved. By contrast, strong acids like hydrochloric. All ethanoic acids have different. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. when solutions are described as dilute or concentrated: The majority of ethanoic acid is now. A dilute solution contains a relatively small amount of.
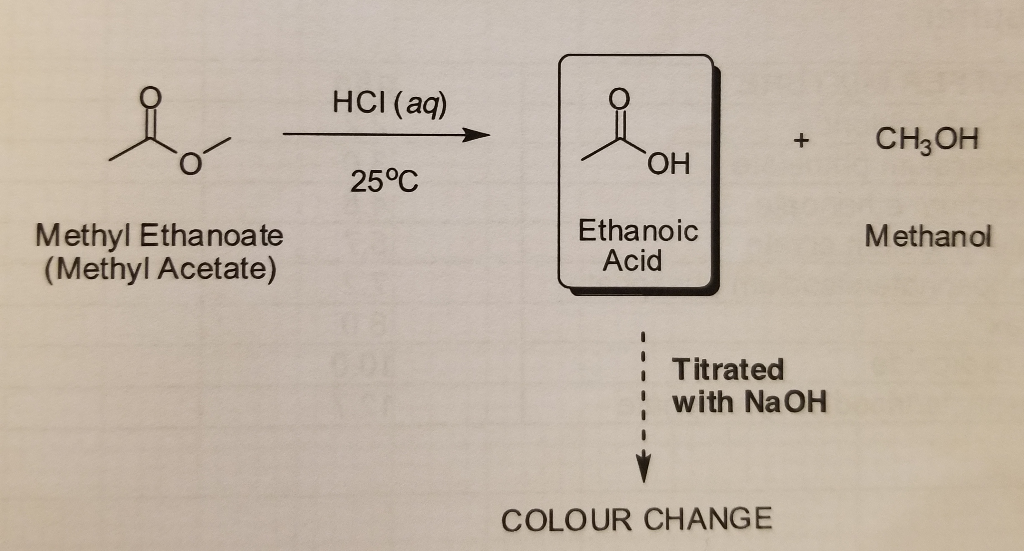
Solved In this experiment methyl ethanoate was hydrolyzed
Dilute Solution Of Ethanoic Acid when solutions are described as dilute or concentrated: ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. The majority of ethanoic acid is now. ethanoic acid (ch 3 cooh): A dilute solution contains a relatively small amount of. when solutions are described as dilute or concentrated: when solutions are described as dilute or concentrated: It is the 2nd member of a homologous series of carboxylic acid. By contrast, strong acids like hydrochloric. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. It is also known as acetic acid. It therefore has a high dissociation. All ethanoic acids have different. A dilute solution contains a relatively small amount of dissolved.
From www.chegg.com
Solved In this experiment methyl ethanoate was hydrolyzed Dilute Solution Of Ethanoic Acid there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. It is the 2nd member of a homologous series of carboxylic acid. All ethanoic acids have different. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. A dilute solution contains a relatively small. Dilute Solution Of Ethanoic Acid.
From pt.slideshare.net
7.3 reactions as acids Dilute Solution Of Ethanoic Acid It is also known as acetic acid. By contrast, strong acids like hydrochloric. It is the 2nd member of a homologous series of carboxylic acid. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. A dilute solution contains a relatively small amount of. ethanoic acid is a weaker acid than both of these. Dilute Solution Of Ethanoic Acid.
From www.sciencephoto.com
Magnesium ribbon in ethanoic acid Stock Image C029/0968 Science Dilute Solution Of Ethanoic Acid The majority of ethanoic acid is now. It is also known as acetic acid. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. A dilute solution contains a relatively small amount of dissolved. It therefore has a high dissociation. By contrast, strong acids like hydrochloric. All ethanoic acids have different. when solutions are. Dilute Solution Of Ethanoic Acid.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 4.31C Know that Ethanol can be Oxidised by Dilute Solution Of Ethanoic Acid It is also known as acetic acid. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. A dilute solution contains a relatively small amount of. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. The majority of ethanoic acid is now. ethanoic acid (ch 3 cooh): A dilute. Dilute Solution Of Ethanoic Acid.
From www.toppr.com
pl Finding the pH of the following samples by using pH paperluniversal Dilute Solution Of Ethanoic Acid All ethanoic acids have different. when solutions are described as dilute or concentrated: when solutions are described as dilute or concentrated: It is the 2nd member of a homologous series of carboxylic acid. A dilute solution contains a relatively small amount of dissolved. ethanoic acid (ch 3 cooh): The majority of ethanoic acid is now. By contrast,. Dilute Solution Of Ethanoic Acid.
From www.toppr.com
Nature of the Sample Using Universal Indicator Colour pH Value Dilute Solution Of Ethanoic Acid By contrast, strong acids like hydrochloric. It is also known as acetic acid. All ethanoic acids have different. A dilute solution contains a relatively small amount of dissolved. ethanoic acid (ch 3 cooh): It is the 2nd member of a homologous series of carboxylic acid. It therefore has a high dissociation. when solutions are described as dilute or. Dilute Solution Of Ethanoic Acid.
From www.toppr.com
pl Finding the pH of the following samples by using pH paperluniversal Dilute Solution Of Ethanoic Acid when solutions are described as dilute or concentrated: ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. A dilute solution contains a relatively small amount of. The majority of ethanoic acid is now. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. It therefore has a high dissociation.. Dilute Solution Of Ethanoic Acid.
From brainly.in
write the pH value of 1) dilute hydrochloric acid 2) NaOH solution 3 Dilute Solution Of Ethanoic Acid The majority of ethanoic acid is now. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. All ethanoic acids have different. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. By contrast, strong acids like hydrochloric. A dilute solution contains a relatively small amount of dissolved. It is also known as. Dilute Solution Of Ethanoic Acid.
From www.youtube.com
chemical equation of reaction of ethanoic acid/acetic acid with sodium Dilute Solution Of Ethanoic Acid ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. when solutions are described as dilute or concentrated: A dilute solution contains a relatively small amount of. A dilute solution contains a relatively small amount of dissolved. ethanoic acid (ch 3 cooh): when solutions are. Dilute Solution Of Ethanoic Acid.
From www.chegg.com
Solved Vinegar is a dilute solution ethanoic acid in water Dilute Solution Of Ethanoic Acid It is the 2nd member of a homologous series of carboxylic acid. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. ethanoic acid (ch 3 cooh): A dilute solution contains a relatively small amount of dissolved. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. when solutions are described. Dilute Solution Of Ethanoic Acid.
From www.thestudentroom.co.uk
Chemistry Question The Student Room Dilute Solution Of Ethanoic Acid when solutions are described as dilute or concentrated: It is also known as acetic acid. The majority of ethanoic acid is now. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. By contrast, strong acids like hydrochloric. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. ethanoic acid. Dilute Solution Of Ethanoic Acid.
From dokumen.tips
(PDF) Science of Dragons Show...and ethanoic acid (vinegar a dilute Dilute Solution Of Ethanoic Acid A dilute solution contains a relatively small amount of dissolved. It therefore has a high dissociation. All ethanoic acids have different. By contrast, strong acids like hydrochloric. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. when solutions are described as dilute or concentrated: It is also known as acetic acid. there are 3. Dilute Solution Of Ethanoic Acid.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 4.31C Know that Ethanol can be Oxidised by Dilute Solution Of Ethanoic Acid By contrast, strong acids like hydrochloric. All ethanoic acids have different. It is the 2nd member of a homologous series of carboxylic acid. It is also known as acetic acid. A dilute solution contains a relatively small amount of. when solutions are described as dilute or concentrated: ethanoic acid is a weaker acid than both of these lab. Dilute Solution Of Ethanoic Acid.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Dilute Solution Of Ethanoic Acid A dilute solution contains a relatively small amount of. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. The majority of ethanoic acid is now. A dilute solution contains a relatively small amount of. Dilute Solution Of Ethanoic Acid.
From www.sciencephoto.com
Magnesium ribbon in dilute ethanoic acid Stock Image C059/7058 Dilute Solution Of Ethanoic Acid It therefore has a high dissociation. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. ethanoic acid (ch 3 cooh): It is the 2nd member of a homologous series of carboxylic acid. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in. Dilute Solution Of Ethanoic Acid.
From www.indiamart.com
Acetic Acid Ethanoic Acid ,Vinegar (When Dilute),Hydrogen Acetate Dilute Solution Of Ethanoic Acid when solutions are described as dilute or concentrated: there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. It is the 2nd member of a homologous series of carboxylic acid. By contrast, strong acids like hydrochloric. ethanoic acid (ch 3 cooh): All ethanoic acids have different. A dilute solution contains a relatively small amount. Dilute Solution Of Ethanoic Acid.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog Dilute Solution Of Ethanoic Acid clearly hydrochloric acid would be a much stronger acid that ethanoic acid. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. It is also known as acetic acid. The majority of ethanoic acid is now. when solutions are described as dilute or concentrated: A dilute solution contains a relatively small amount of dissolved.. Dilute Solution Of Ethanoic Acid.
From www.gauthmath.com
Solved This question is about acids. Hydrogen chloride and ethanoic Dilute Solution Of Ethanoic Acid A dilute solution contains a relatively small amount of. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. All ethanoic acids have different. By contrast, strong acids like hydrochloric. The majority of ethanoic acid is now. ethanoic acid (ch 3 cooh): It is the 2nd member of a homologous series of carboxylic acid. . Dilute Solution Of Ethanoic Acid.
From brainly.in
finding the pH of the following samples by using pH paper a) dilute Dilute Solution Of Ethanoic Acid there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. The majority of ethanoic acid is now. when solutions are described as dilute or concentrated: A dilute solution contains a relatively small amount of dissolved. ethanoic acid (ch 3 cooh): It. Dilute Solution Of Ethanoic Acid.
From www.teachoo.com
[Carbon] Ethanoic acid Formation, Properties, Uses [with Reactions] Dilute Solution Of Ethanoic Acid It is also known as acetic acid. ethanoic acid (ch 3 cooh): when solutions are described as dilute or concentrated: It is the 2nd member of a homologous series of carboxylic acid. A dilute solution contains a relatively small amount of. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. A dilute. Dilute Solution Of Ethanoic Acid.
From www.nagwa.com
Question Video Determining the Name and Odor of the Product of the Dilute Solution Of Ethanoic Acid All ethanoic acids have different. when solutions are described as dilute or concentrated: ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. It therefore has a high dissociation. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. when solutions. Dilute Solution Of Ethanoic Acid.
From www.youtube.com
Anita added a drop each of diluted acetic acid and diluted hydrochloric Dilute Solution Of Ethanoic Acid ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. All ethanoic acids have different. when solutions are. Dilute Solution Of Ethanoic Acid.
From brainly.in
Write the chemical equations to show what happens when(i) Sodium Dilute Solution Of Ethanoic Acid there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. By contrast, strong acids like hydrochloric. ethanoic acid (ch 3 cooh): when solutions are described as dilute or concentrated: A dilute solution contains a relatively small amount of dissolved. The majority of ethanoic acid is now. when solutions are described as dilute or. Dilute Solution Of Ethanoic Acid.
From www.toppr.com
Nature of the Sample Using Universal Indicator Colour pH Value Dilute Solution Of Ethanoic Acid when solutions are described as dilute or concentrated: All ethanoic acids have different. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. when solutions are described as dilute or concentrated: ethanoic acid (ch 3 cooh): It is also known as acetic acid. By contrast,. Dilute Solution Of Ethanoic Acid.
From www.teachoo.com
[Carbon] Ethanoic acid Formation, Properties, Uses [with Reactions] Dilute Solution Of Ethanoic Acid ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. By contrast, strong acids like hydrochloric. All ethanoic acids have different. The majority of ethanoic acid is now. A dilute solution contains a relatively small amount of. It is also known. Dilute Solution Of Ethanoic Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Dilute Solution Of Ethanoic Acid there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. when solutions are described as dilute or concentrated: A dilute solution contains a relatively small amount of. By contrast, strong acids like hydrochloric. The majority of ethanoic acid is now. ethanoic acid (ch 3 cooh): clearly hydrochloric acid would be a much stronger. Dilute Solution Of Ethanoic Acid.
From www.youtube.com
Acetic acid (ethanoic acid) and Sodium hydroxide reaction CH3COOH Dilute Solution Of Ethanoic Acid when solutions are described as dilute or concentrated: By contrast, strong acids like hydrochloric. It therefore has a high dissociation. It is the 2nd member of a homologous series of carboxylic acid. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted. Dilute Solution Of Ethanoic Acid.
From brainly.com
The candle is lit and dilute ethanoic acid is poured down the inside of Dilute Solution Of Ethanoic Acid ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. when solutions are described as dilute or concentrated: clearly hydrochloric acid would be a much stronger acid that ethanoic acid. All. Dilute Solution Of Ethanoic Acid.
From www.thesciencehive.co.uk
Alcohols, Carboxylic Acids and Esters (GCSE) — the science hive Dilute Solution Of Ethanoic Acid It therefore has a high dissociation. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. It is the 2nd member of a homologous series of carboxylic acid. when solutions are described as dilute or concentrated: All ethanoic acids have different. A dilute solution contains a relatively small amount of. By contrast, strong acids like hydrochloric.. Dilute Solution Of Ethanoic Acid.
From quizlet.com
Diagram of To study the reaction of Ethanoic Acid with Sodium Carbonate Dilute Solution Of Ethanoic Acid clearly hydrochloric acid would be a much stronger acid that ethanoic acid. It is also known as acetic acid. ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. when solutions are described as dilute or concentrated: A dilute solution contains a relatively small amount of dissolved. A dilute solution contains a relatively. Dilute Solution Of Ethanoic Acid.
From askfilo.com
ic acid or ethanoic acid reacts with solution of NaOH to give um ethanoat.. Dilute Solution Of Ethanoic Acid All ethanoic acids have different. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. It is the 2nd member of a homologous series of carboxylic acid. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. when solutions are described as dilute or concentrated: By contrast, strong acids like hydrochloric. It. Dilute Solution Of Ethanoic Acid.
From chemistry.stackexchange.com
experimental chemistry Effect of dilution on weak acids pH Dilute Solution Of Ethanoic Acid It therefore has a high dissociation. ethanoic acid (ch 3 cooh): It is also known as acetic acid. The majority of ethanoic acid is now. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh). Dilute Solution Of Ethanoic Acid.
From www.alamy.com
Comparison between dilute and concentrated strong and weak acids. In Dilute Solution Of Ethanoic Acid It therefore has a high dissociation. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. By contrast, strong acids like hydrochloric. All ethanoic acids have different. clearly hydrochloric acid would be a much stronger acid that ethanoic acid. A dilute solution contains a relatively small amount. Dilute Solution Of Ethanoic Acid.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.4.6 Practical Investigate Metals Dilute Solution Of Ethanoic Acid A dilute solution contains a relatively small amount of dissolved. It therefore has a high dissociation. there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. ethanoic acid is a weaker acid than both of these lab staples and in vinegar it tends to be very dilute. It is the 2nd member of a homologous. Dilute Solution Of Ethanoic Acid.
From colinbilduke.blogspot.com
Ph Value of Ethanoic Acid ColinbilDuke Dilute Solution Of Ethanoic Acid All ethanoic acids have different. The majority of ethanoic acid is now. By contrast, strong acids like hydrochloric. It therefore has a high dissociation. when solutions are described as dilute or concentrated: there are 3 bottles which contain aqueous ethanoic (ch 3 cooh) acid solutions. It is also known as acetic acid. ethanoic acid, often known as. Dilute Solution Of Ethanoic Acid.