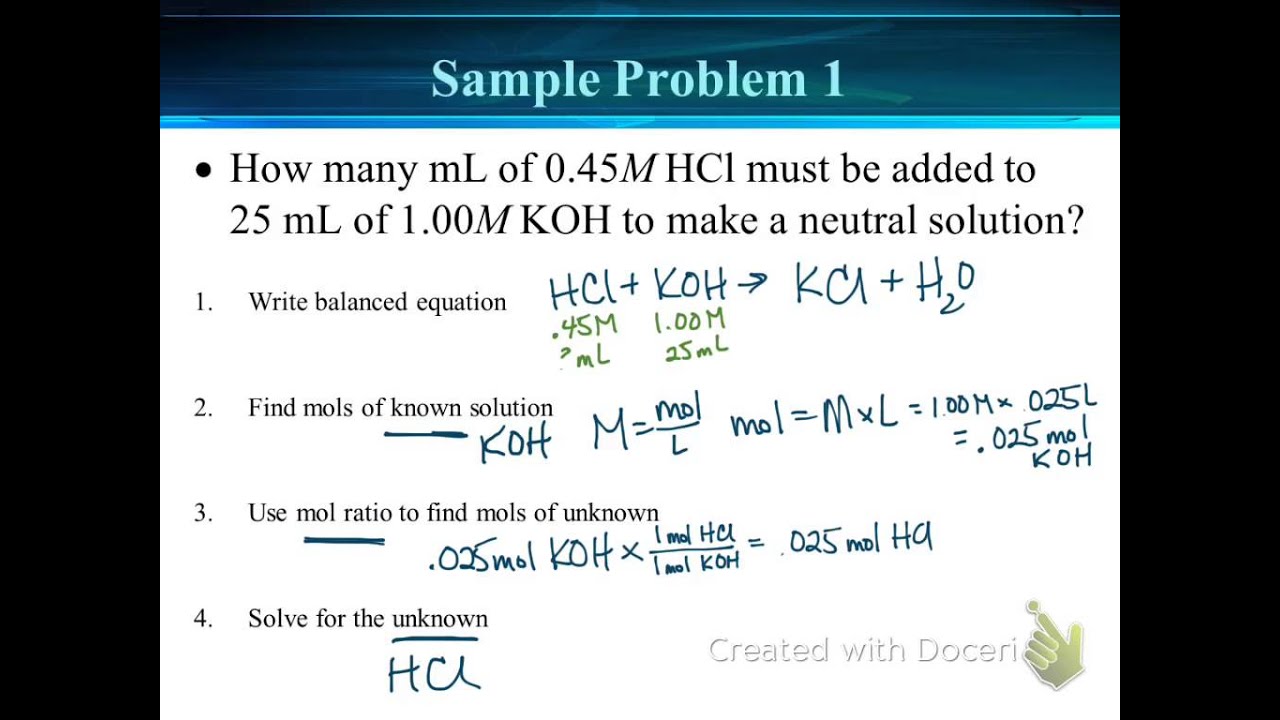
Titration Example Problems With Answers . the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. find the requested quantities in the following problems: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. answers to the titrations practice worksheet. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. For questions 1 and 2, the units for your final answer.
from www.youtube.com
If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. answers to the titrations practice worksheet. For questions 1 and 2, the units for your final answer. find the requested quantities in the following problems: List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using.
Solving AcidBase Titration Problems YouTube
Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. For questions 1 and 2, the units for your final answer. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. answers to the titrations practice worksheet. find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Titration Example Problems With Answers A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. answers to the titrations practice worksheet. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution,. Titration Example Problems With Answers.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Example Problems With Answers find the requested quantities in the following problems: answers to the titrations practice worksheet. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. in this section, we will see how to perform. Titration Example Problems With Answers.
From learningschoolmurgkerny1.z13.web.core.windows.net
Titration Practical Questions And Answers Pdf Titration Example Problems With Answers find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. For questions 1 and 2, the units for your final answer. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. the example below demonstrates the. Titration Example Problems With Answers.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Example Problems With Answers answers to the titrations practice worksheet. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. For questions 1 and 2,. Titration Example Problems With Answers.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Titration Example Problems With Answers If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. answers to the titrations practice worksheet. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. the example below demonstrates the technique. Titration Example Problems With Answers.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Titration Example Problems With Answers List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. answers to the titrations practice worksheet. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. the. Titration Example Problems With Answers.
From studylib.net
Titration Practice Worksheet Titration Example Problems With Answers If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. For questions 1 and 2, the units for your final answer. answers to the titrations practice worksheet. the example below demonstrates the technique to. Titration Example Problems With Answers.
From www.chegg.com
Solved PART B Titrations Introduction for part B, three Titration Example Problems With Answers A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or. Titration Example Problems With Answers.
From www.youtube.com
Titration (sample problems) YouTube Titration Example Problems With Answers find the requested quantities in the following problems: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. answers to the titrations practice worksheet. For questions 1 and 2, the units for your final answer. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution,. Titration Example Problems With Answers.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Titration Example Problems With Answers answers to the titrations practice worksheet. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. find the requested quantities in the following problems: For questions 1 and 2, the units for your final answer. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved. Titration Example Problems With Answers.
From childhealthpolicy.vumc.org
😱 Titration lab report discussion. Lab Report 9. 20221018 Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. answers to the titrations practice worksheet. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. the example below demonstrates the technique to. Titration Example Problems With Answers.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Example Problems With Answers List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. find the requested quantities in the following problems: in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using.. Titration Example Problems With Answers.
From mungfali.com
Acid Base Titration Calculation Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. answers to the titrations practice worksheet. the example. Titration Example Problems With Answers.
From www.chegg.com
Solved To Review How Titration Calculations Work, Here's Titration Example Problems With Answers If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to. Titration Example Problems With Answers.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml. Titration Example Problems With Answers.
From learningzonelisannin.z14.web.core.windows.net
Chemistry Titration Questions And Answers Titration Example Problems With Answers A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. answers to the titrations practice worksheet. For questions 1 and 2, the units for your final answer. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. find the. Titration Example Problems With Answers.
From tukioka-clinic.com
️ Acid base titration problems with answers pdf. Eleventh grade Lesson Titration Example Problems With Answers List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. answers to the titrations practice worksheet. in this section, we will see how to perform. Titration Example Problems With Answers.
From materiallistdedmon.z22.web.core.windows.net
Gas Law Stoichiometry Worksheets Titration Example Problems With Answers A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. find the requested quantities in the following problems: in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. If it takes 54 ml of 0.1. Titration Example Problems With Answers.
From dxodobzvw.blob.core.windows.net
Titration Lab Follow Up Questions at Wen blog Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia. Titration Example Problems With Answers.
From www.chem.fsu.edu
Quantitative Analysis Titration Example Problems With Answers List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. answers to the titrations practice worksheet. in this section, we will see how to perform calculations to. Titration Example Problems With Answers.
From dxooyumci.blob.core.windows.net
Titration Practice Problems Chemistry at Gloria Ogorman blog Titration Example Problems With Answers the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. find the requested quantities in the following problems: answers to the titrations practice worksheet. If it takes 54 ml of 0.1 m. Titration Example Problems With Answers.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Example Problems With Answers the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. answers to the titrations practice. Titration Example Problems With Answers.
From worksheets.clipart-library.com
Titration Problems worksheet Live Worksheets Worksheets Library Titration Example Problems With Answers List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. . Titration Example Problems With Answers.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. the example below demonstrates the technique to solve a titration problem for a titration. Titration Example Problems With Answers.
From chp090.chemistry.wustl.edu
Redox Titration Problem Titration Example Problems With Answers List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. answers to the titrations practice worksheet. find the requested quantities in the following problems: For. Titration Example Problems With Answers.
From www.youtube.com
9.5b Titration Example Problems YouTube Titration Example Problems With Answers find the requested quantities in the following problems: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. answers to the titrations practice worksheet. List the major species at points a, b,. Titration Example Problems With Answers.
From mungfali.com
Titration Example Problem Titration Example Problems With Answers If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. For questions 1 and 2, the units for your final answer. find the requested quantities in the following. Titration Example Problems With Answers.
From www.chegg.com
Solved Titrations Practice WorksheetFxtra practice Find the Titration Example Problems With Answers in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. For questions 1 and 2, the units for your final answer. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. List. Titration Example Problems With Answers.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Example Problems With Answers A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. in this section, we will see how to perform calculations to predict the ph at any point in a titration. Titration Example Problems With Answers.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Example Problems With Answers answers to the titrations practice worksheet. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. find the requested quantities in the. Titration Example Problems With Answers.
From tukioka-clinic.com
👍 Solve titration problems. How to solve titration problems step by Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. A 1.0000 gram sample of k2co3. Titration Example Problems With Answers.
From www.vrogue.co
Acid Base Titration Problems Basic Introduction Calcu vrogue.co Titration Example Problems With Answers find the requested quantities in the following problems: the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. in this section, we will see how. Titration Example Problems With Answers.
From learningschoolmysticis9.z22.web.core.windows.net
Chemistry Titration Questions And Answers Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. find the requested quantities in the following problems: the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. A 1.0000. Titration Example Problems With Answers.
From printablelibfinance.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Titration Example Problems With Answers For questions 1 and 2, the units for your final answer. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what. A 1.0000 gram sample of k2co3 (138.2055 g/mol). Titration Example Problems With Answers.
From studylib.net
acid base titration worksheet answer key Titration Example Problems With Answers answers to the titrations practice worksheet. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. For questions 1 and 2,. Titration Example Problems With Answers.