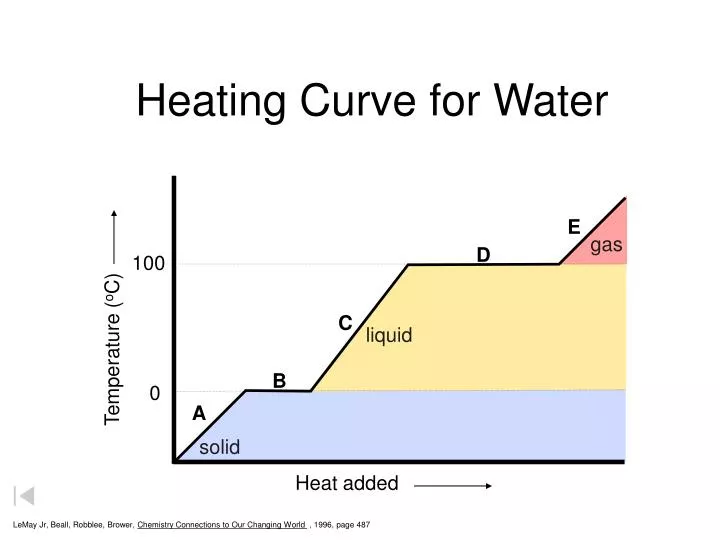
Heating Curve Labeled Heat Of Fusion . Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. A heating curve of a substance shows the relationship of temperature, state of matter, and heat. When studying chemistry, “fusion” simply has the same definition as melting. define heat of fusion and heat of vaporization; The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Thus the actual length of line will be \({n \times. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? what is heat of fusion? Relate heat of fusion and heat of vaporization to heat of freezing and.
from www.slideserve.com
what is heat of fusion? Thus the actual length of line will be \({n \times. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? Relate heat of fusion and heat of vaporization to heat of freezing and. A heating curve of a substance shows the relationship of temperature, state of matter, and heat. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. define heat of fusion and heat of vaporization; Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. When studying chemistry, “fusion” simply has the same definition as melting. The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the.
PPT Heating Curve for Water PowerPoint Presentation, free download
Heating Curve Labeled Heat Of Fusion Thus the actual length of line will be \({n \times. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Thus the actual length of line will be \({n \times. The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. When studying chemistry, “fusion” simply has the same definition as melting. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? A heating curve of a substance shows the relationship of temperature, state of matter, and heat. what is heat of fusion? define heat of fusion and heat of vaporization; Relate heat of fusion and heat of vaporization to heat of freezing and.
From exorgxbax.blob.core.windows.net
Heating Curve Graph Fusion at Stephen Cooks blog Heating Curve Labeled Heat Of Fusion Relate heat of fusion and heat of vaporization to heat of freezing and. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Thus the actual length of line will be \({n \times. When studying chemistry, “fusion” simply has the. Heating Curve Labeled Heat Of Fusion.
From learningfullmaurer.z1.web.core.windows.net
Heating Curve Of Water Heating Curve Labeled Heat Of Fusion what is heat of fusion? Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. Relate heat of fusion and heat of vaporization to heat of freezing and. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required. Heating Curve Labeled Heat Of Fusion.
From www.chegg.com
Solved The Figure shown below is the heating curve obtained Heating Curve Labeled Heat Of Fusion Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? define heat of fusion and heat of vaporization; Relate heat of fusion. Heating Curve Labeled Heat Of Fusion.
From www.showme.com
Heating and Cooling Curves Explained Science, Heating Curve, Cooling Heating Curve Labeled Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? what is heat. Heating Curve Labeled Heat Of Fusion.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Labeled Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. Thus the actual length of line will be \({n \times. what is heat of fusion? Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Recall the relationship. Heating Curve Labeled Heat Of Fusion.
From www.youtube.com
Heating and Cooling Curves IGCSE/ O level Chemistry / lec6 Chapter1 Heating Curve Labeled Heat Of Fusion define heat of fusion and heat of vaporization; Thus the actual length of line will be \({n \times. When studying chemistry, “fusion” simply has the same definition as melting. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure.. Heating Curve Labeled Heat Of Fusion.
From worksheetfullfunniest.z21.web.core.windows.net
What Is A Heat Curve Heating Curve Labeled Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. A heating curve of a substance shows the relationship of temperature, state of matter, and heat. Heat of fusion, also called enthalpy of fusion or latent heat of. Heating Curve Labeled Heat Of Fusion.
From exorgxbax.blob.core.windows.net
Heating Curve Graph Fusion at Stephen Cooks blog Heating Curve Labeled Heat Of Fusion Relate heat of fusion and heat of vaporization to heat of freezing and. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat. Heating Curve Labeled Heat Of Fusion.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curve Labeled Heat Of Fusion define heat of fusion and heat of vaporization; Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,.. Heating Curve Labeled Heat Of Fusion.
From classdbhart.z21.web.core.windows.net
Heating And Cooling Curves Chemistry Heating Curve Labeled Heat Of Fusion define heat of fusion and heat of vaporization; A heating curve of a substance shows the relationship of temperature, state of matter, and heat. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? When studying chemistry, “fusion” simply has the same definition. Heating Curve Labeled Heat Of Fusion.
From study.com
Heating & Cooling Curves Definition, Phases & Examples Lesson Heating Curve Labeled Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? define heat of. Heating Curve Labeled Heat Of Fusion.
From www.slideserve.com
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation Heating Curve Labeled Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. what is heat of fusion? if we are told that water's heat of fusion (δhfus) is 6kj/mol,. Heating Curve Labeled Heat Of Fusion.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Labeled Heat Of Fusion what is heat of fusion? When studying chemistry, “fusion” simply has the same definition as melting. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? A heating curve of a substance shows the relationship of temperature, state of matter, and heat. . Heating Curve Labeled Heat Of Fusion.
From preparatorychemistry.com
Heating Curve Heating Curve Labeled Heat Of Fusion what is heat of fusion? define heat of fusion and heat of vaporization; Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles. Heating Curve Labeled Heat Of Fusion.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Labeled Heat Of Fusion Thus the actual length of line will be \({n \times. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? what is heat of fusion? Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying. Heating Curve Labeled Heat Of Fusion.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1540635 Heating Curve Labeled Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Relate heat of fusion and heat of vaporization to heat of freezing and. A heating curve of a substance shows the relationship of temperature, state of matter, and heat. The. Heating Curve Labeled Heat Of Fusion.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve Labeled Heat Of Fusion Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. define heat of fusion and heat of vaporization; The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. Thus the actual length of line will be \({n \times. Relate heat of. Heating Curve Labeled Heat Of Fusion.
From quizlet.com
Heating Curve Diagram Quizlet Heating Curve Labeled Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? Relate heat of fusion and heat of vaporization to heat of freezing and. Heat of fusion, also called enthalpy of fusion or latent. Heating Curve Labeled Heat Of Fusion.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Labeled Heat Of Fusion what is heat of fusion? A heating curve of a substance shows the relationship of temperature, state of matter, and heat. The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. Thus the actual length of line will be \({n \times. define heat of fusion and heat of vaporization; When studying. Heating Curve Labeled Heat Of Fusion.
From ch301.cm.utexas.edu
heating curve Heating Curve Labeled Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. Relate heat of fusion and heat of vaporization to heat of freezing and. what is heat of fusion? Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. if we are told that water's heat of. Heating Curve Labeled Heat Of Fusion.
From www.youtube.com
Heating Curve Discussion YouTube Heating Curve Labeled Heat Of Fusion The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. Thus the actual length of line will be \({n \times. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. define heat of fusion and heat of vaporization; When studying chemistry,. Heating Curve Labeled Heat Of Fusion.
From chem.libretexts.org
5.5.1 Heating Curves and Phase Changes (Problems) Chemistry LibreTexts Heating Curve Labeled Heat Of Fusion A heating curve of a substance shows the relationship of temperature, state of matter, and heat. When studying chemistry, “fusion” simply has the same definition as melting. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. Heat of fusion, also called enthalpy of fusion or latent heat of. Heating Curve Labeled Heat Of Fusion.
From curiophysics.com
Heating Curve » Curio Physics Heating Curve Labeled Heat Of Fusion Thus the actual length of line will be \({n \times. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. define heat of fusion and heat of vaporization; A heating curve of a substance shows the relationship of temperature, state of matter, and heat. Heat of fusion, also. Heating Curve Labeled Heat Of Fusion.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curve Labeled Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. what is heat of fusion? Relate heat of fusion and heat of vaporization to heat of freezing and. The processes on the right side of figure \ (\pageindex {1}\)—freezing,. Heating Curve Labeled Heat Of Fusion.
From exorgxbax.blob.core.windows.net
Heating Curve Graph Fusion at Stephen Cooks blog Heating Curve Labeled Heat Of Fusion define heat of fusion and heat of vaporization; what is heat of fusion? Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. When studying chemistry, “fusion” simply has the same definition as melting. Thus the actual length. Heating Curve Labeled Heat Of Fusion.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Heating Curve Labeled Heat Of Fusion Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. define heat of fusion and heat of vaporization; if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? The processes on the. Heating Curve Labeled Heat Of Fusion.
From mavink.com
What Is A Heating Curve Heating Curve Labeled Heat Of Fusion The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. what is heat of fusion? define heat of fusion and heat of vaporization; Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of. Heating Curve Labeled Heat Of Fusion.
From courses.lumenlearning.com
Phase Transitions General Chemistry Heating Curve Labeled Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. A heating curve of a substance shows the relationship of temperature, state of matter, and heat. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. what is heat of fusion? Thus the actual length of line. Heating Curve Labeled Heat Of Fusion.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes Chemistry LibreTexts Heating Curve Labeled Heat Of Fusion The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. A heating curve of a substance shows the relationship of temperature, state of matter, and heat. Thus the actual length of line will be \({n \times. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat. Heating Curve Labeled Heat Of Fusion.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Labeled Heat Of Fusion Relate heat of fusion and heat of vaporization to heat of freezing and. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. what is heat of fusion? if we are told that water's heat of fusion (δhfus). Heating Curve Labeled Heat Of Fusion.
From www.slideserve.com
PPT The Heating Curve PowerPoint Presentation, free download ID5070642 Heating Curve Labeled Heat Of Fusion A heating curve of a substance shows the relationship of temperature, state of matter, and heat. Relate heat of fusion and heat of vaporization to heat of freezing and. if we are told that water's heat of fusion (δhfus) is 6kj/mol, then how much heat would be required to melt 5 moles of water? Recall the relationship between the. Heating Curve Labeled Heat Of Fusion.
From www.youtube.com
Heating Curve Practice 3 Step with Phase Change Fusion YouTube Heating Curve Labeled Heat Of Fusion what is heat of fusion? The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Relate heat of fusion and heat of. Heating Curve Labeled Heat Of Fusion.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Curve Labeled Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. Relate heat of fusion and heat of vaporization to heat of freezing and. A heating curve of a substance shows the relationship of temperature, state of matter, and heat. Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change,. Heating Curve Labeled Heat Of Fusion.
From dxohghvna.blob.core.windows.net
Heating Curve Lesson at Sarah Ables blog Heating Curve Labeled Heat Of Fusion Recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, δt,. define heat of fusion and heat of vaporization; Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure.. Heating Curve Labeled Heat Of Fusion.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Curve Labeled Heat Of Fusion Relate heat of fusion and heat of vaporization to heat of freezing and. The processes on the right side of figure \ (\pageindex {1}\)—freezing, condensation, and deposition, which are the. When studying chemistry, “fusion” simply has the same definition as melting. what is heat of fusion? Recall the relationship between the amount of heat absorbed or released by a. Heating Curve Labeled Heat Of Fusion.