Fda Drug Container Labeling Requirements . Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. It provides a set of. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and.
from www.lifealert.org
(a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. It provides a set of.
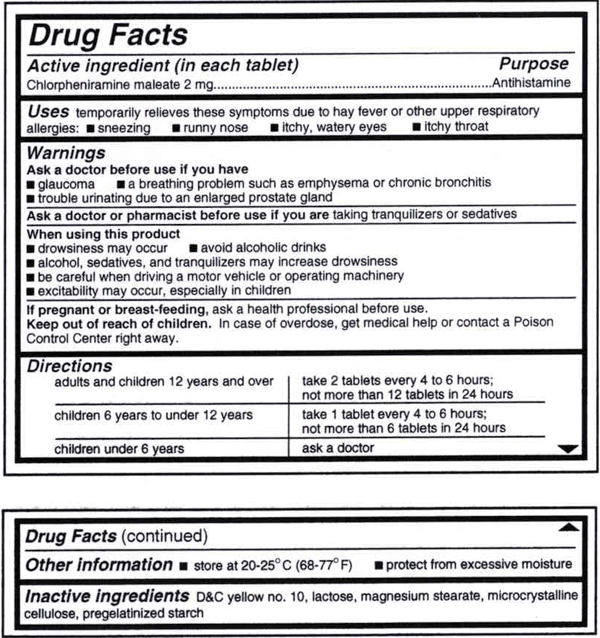
OvertheCounter Medicine Label
Fda Drug Container Labeling Requirements This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. It provides a set of. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Fda Drug Container Labeling Requirements (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. It provides a set of. Label has the meaning. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements It provides a set of. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. It provides a set of. Fda Drug Container Labeling Requirements.
From www.greenlight.guru
FDA Medical Device Labeling Checklist [Free Download] Fda Drug Container Labeling Requirements It provides a set of. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. (1) permit a statement. Fda Drug Container Labeling Requirements.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. It provides a set of. (a) the symbol requirements of 1302.03. Fda Drug Container Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Drug Container Labeling Requirements (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. This guidance focuses on safety aspects of the application. Fda Drug Container Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Drug Container Labeling Requirements (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. It provides a set of. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and.. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. It provides a set of.. Fda Drug Container Labeling Requirements.
From www.artworkflowhq.com
The Ultimate 2023 Guide to FDA Cosmetic Labeling Regulations Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. It provides a set of. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. Label has the meaning set forth in. Fda Drug Container Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. This guidance focuses on safety aspects of the application holder’s container. Fda Drug Container Labeling Requirements.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Drug Container Labeling Requirements It provides a set of. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling. Fda Drug Container Labeling Requirements.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Drug Container Labeling Requirements Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. It provides a set of principles and recommendations for ensuring that. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (1) permit a statement of differences. Fda Drug Container Labeling Requirements.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. Label has the meaning set forth in section 201. Fda Drug Container Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. This guidance focuses on safety aspects of the application holder’s container. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. It provides a set of. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. (1) permit a statement. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. It provides a set of. This guidance focuses on. Fda Drug Container Labeling Requirements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Fda Drug Container Labeling Requirements It provides a set of. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container. Fda Drug Container Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. (a) the symbol requirements of 1302.03 through 1302.05 apply to. Fda Drug Container Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Drug Container Labeling Requirements Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. This guidance focuses on safety aspects of the application holder’s. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements It provides a set of. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. It provides. Fda Drug Container Labeling Requirements.
From www.fda.gov
OTC Drug Facts Label FDA Fda Drug Container Labeling Requirements This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. It provides a set of principles and recommendations for ensuring that. Fda Drug Container Labeling Requirements.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. It provides a set of.. Fda Drug Container Labeling Requirements.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. This guidance focuses on safety aspects of the application holder’s container. Fda Drug Container Labeling Requirements.
From mavink.com
Parts Of Drug Label Fda Drug Container Labeling Requirements It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. It provides a set of. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse.. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. Label has the meaning set forth in section. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. (1) permit a statement of. Fda Drug Container Labeling Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. (a) the symbol requirements of 1302.03 through 1302.05 apply. Fda Drug Container Labeling Requirements.
From loeaqczrf.blob.core.windows.net
Guidelines Labelling Pharmaceutical Products at Ralph Swanson blog Fda Drug Container Labeling Requirements This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (a) the symbol requirements of 1302.03 through 1302.05 apply to. Fda Drug Container Labeling Requirements.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (1) permit a statement of differences of opinion with respect to. Fda Drug Container Labeling Requirements.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Drug Container Labeling Requirements (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. (1) permit a statement of differences of. Fda Drug Container Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. It provides a set of. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (1). Fda Drug Container Labeling Requirements.
From www.lifealert.org
OvertheCounter Medicine Label Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (a) the symbol requirements of 1302.03 through. Fda Drug Container Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Drug Container Labeling Requirements (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all. Label has the meaning set forth in section 201 (k) of. Fda Drug Container Labeling Requirements.
From packaginghub.com
FDA Packaging and Labeling Requirements Guide Packaging Hub Fda Drug Container Labeling Requirements It provides a set of principles and recommendations for ensuring that critical elements of a product’s container label and. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions,. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. It provides a set of. (1). Fda Drug Container Labeling Requirements.
From www.finnegan.com
Final FDA Guidance on Safety Considerations for Medication Container Fda Drug Container Labeling Requirements This guidance focuses on safety aspects of the application holder’s container label and carton labeling design. (1) permit a statement of differences of opinion with respect to warnings (including contraindications, precautions, adverse. (a) the symbol requirements of 1302.03 through 1302.05 apply to every commercial container containing, and to all labeling of, controlled. Label has the meaning set forth in section. Fda Drug Container Labeling Requirements.