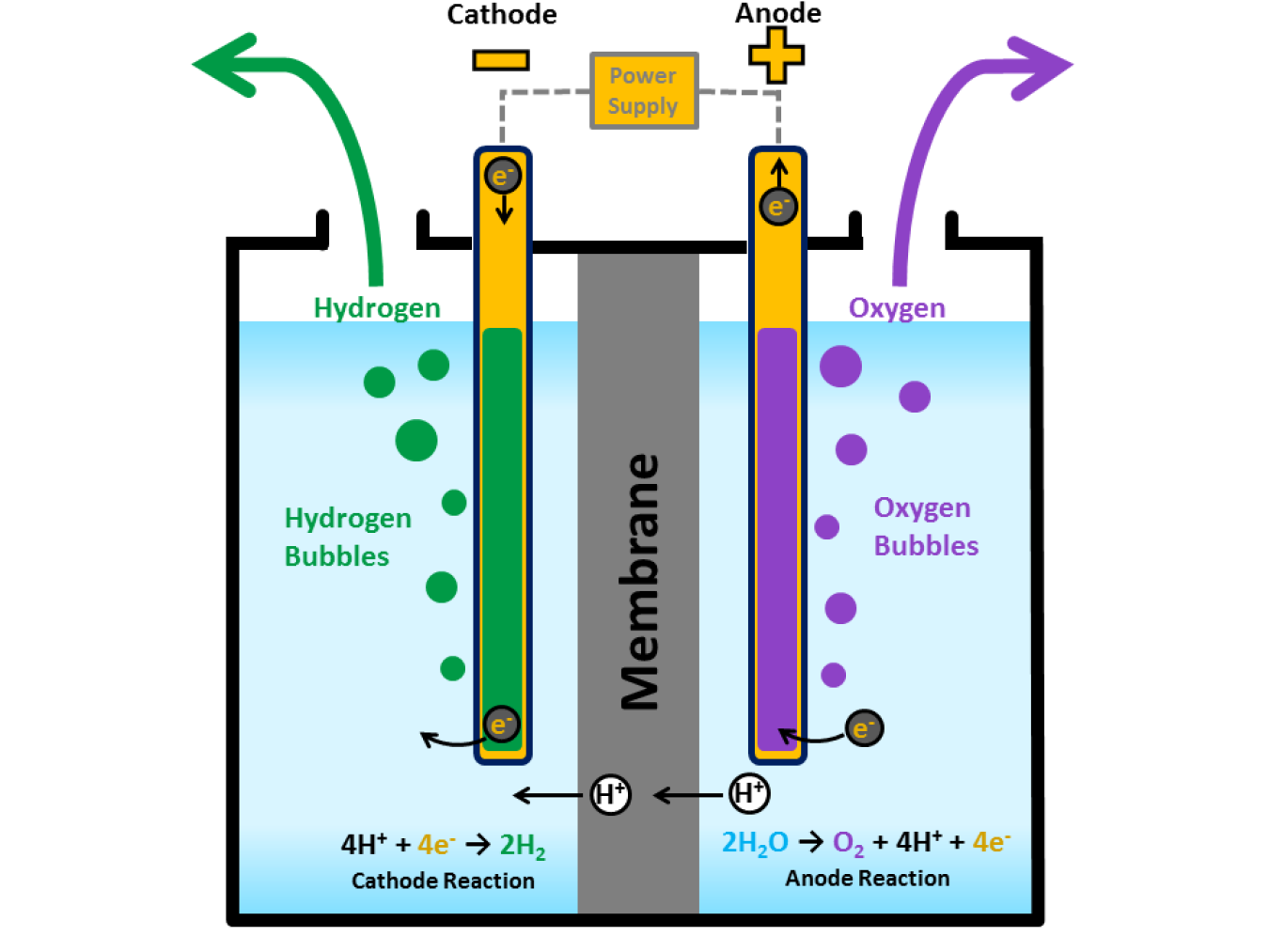
Why Are Carbon Electrodes Used In Electrolysis . the electrolysis of sodium chloride solution using carbon electrodes. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. the electrolysis of aqueous solutions of ionic compounds using inert electrodes. This page looks in detail at the electrolysis of solutions of ionic. Sodium is well above hydrogen in the electrochemical series and so, using the. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. Graphite (a form of carbon) and platinum. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. they just provide a surface for ions to gain or lose electrons so they form products.
from www.energy.gov
the electrolysis of sodium chloride solution using carbon electrodes. they just provide a surface for ions to gain or lose electrons so they form products. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. Graphite (a form of carbon) and platinum. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. This page looks in detail at the electrolysis of solutions of ionic. the electrolysis of aqueous solutions of ionic compounds using inert electrodes. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.
Hydrogen Production Electrolysis Department of Energy
Why Are Carbon Electrodes Used In Electrolysis they just provide a surface for ions to gain or lose electrons so they form products. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. the electrolysis of aqueous solutions of ionic compounds using inert electrodes. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. Sodium is well above hydrogen in the electrochemical series and so, using the. they just provide a surface for ions to gain or lose electrons so they form products. the electrolysis of sodium chloride solution using carbon electrodes. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. This page looks in detail at the electrolysis of solutions of ionic. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Graphite (a form of carbon) and platinum.
From www.mbrashem.com
Why Are Carbon Electrodes Used in Electrolysis? M. Brashem, Inc Why Are Carbon Electrodes Used In Electrolysis the electrolysis of sodium chloride solution using carbon electrodes. Sodium is well above hydrogen in the electrochemical series and so, using the. they just provide a surface for ions to gain or lose electrons so they form products. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. Graphite. Why Are Carbon Electrodes Used In Electrolysis.
From chemistry.stackexchange.com
electrochemistry Standard electrode potential for electrolytic vs Why Are Carbon Electrodes Used In Electrolysis this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. they just provide a surface for ions to gain or lose electrons so they form products. This page looks in detail at the electrolysis of solutions of ionic. the electrolysis of sodium chloride solution using carbon electrodes. Graphite (a form of carbon). Why Are Carbon Electrodes Used In Electrolysis.
From exovumdtp.blob.core.windows.net
How Is Electrolysis Done at Margaret Newman blog Why Are Carbon Electrodes Used In Electrolysis the electrolysis of sodium chloride solution using carbon electrodes. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. Graphite (a form of carbon) and platinum. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. Sodium is. Why Are Carbon Electrodes Used In Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Why Are Carbon Electrodes Used In Electrolysis they just provide a surface for ions to gain or lose electrons so they form products. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. Sodium is well above hydrogen in the electrochemical series and so, using the. carbon electrodes are used in electrolysis due to their competence as a conductor and. Why Are Carbon Electrodes Used In Electrolysis.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Why Are Carbon Electrodes Used In Electrolysis the electrolysis of sodium chloride solution using carbon electrodes. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free.. Why Are Carbon Electrodes Used In Electrolysis.
From courses.lumenlearning.com
17.2 Galvanic Cells Chemistry Why Are Carbon Electrodes Used In Electrolysis the electrolysis of sodium chloride solution using carbon electrodes. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. the electrolysis of aqueous solutions of ionic compounds. Why Are Carbon Electrodes Used In Electrolysis.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Why Are Carbon Electrodes Used In Electrolysis the electrolysis of aqueous solutions of ionic compounds using inert electrodes. Sodium is well above hydrogen in the electrochemical series and so, using the. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity. Why Are Carbon Electrodes Used In Electrolysis.
From blog.thepipingmart.com
Why are carbon electrodes used in electrolysis? Why Are Carbon Electrodes Used In Electrolysis the electrolysis of sodium chloride solution using carbon electrodes. Graphite (a form of carbon) and platinum. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. This page looks in detail at the electrolysis of solutions of ionic. carbon electrodes have the bulk amount of free electrons that are free for transfer. Why Are Carbon Electrodes Used In Electrolysis.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Why Are Carbon Electrodes Used In Electrolysis carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. the electrolysis of aqueous solutions of ionic compounds using inert electrodes. This page looks in detail at the electrolysis of solutions of ionic. the electrolysis of sodium chloride solution using carbon electrodes. Sodium is well above hydrogen in the. Why Are Carbon Electrodes Used In Electrolysis.
From www.elevise.co.uk
C4 J) Electrolysis Part 1 AQA Combined Science Trilogy Elevise Why Are Carbon Electrodes Used In Electrolysis carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. the electrolysis of aqueous solutions of ionic compounds using inert electrodes. Graphite (a form of carbon) and platinum. This page looks in detail at the. Why Are Carbon Electrodes Used In Electrolysis.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Why Are Carbon Electrodes Used In Electrolysis in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. This page looks in detail at the electrolysis. Why Are Carbon Electrodes Used In Electrolysis.
From brainly.in
electrolysis of acidified water using platinum electrodes gives Why Are Carbon Electrodes Used In Electrolysis this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. the electrolysis of sodium chloride solution using carbon electrodes. the electrolysis of aqueous solutions of ionic compounds using inert electrodes.. Why Are Carbon Electrodes Used In Electrolysis.
From issuu.com
Use of Carbon Electrode in Electrolytes by dekresearch Issuu Why Are Carbon Electrodes Used In Electrolysis Sodium is well above hydrogen in the electrochemical series and so, using the. This page looks in detail at the electrolysis of solutions of ionic. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free.. Why Are Carbon Electrodes Used In Electrolysis.
From blog.thepipingmart.com
Why are carbon electrodes used in electrolysis? Why Are Carbon Electrodes Used In Electrolysis carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. the electrolysis of aqueous solutions of ionic compounds using inert electrodes. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Sodium is well above hydrogen in the electrochemical. Why Are Carbon Electrodes Used In Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Why Are Carbon Electrodes Used In Electrolysis This page looks in detail at the electrolysis of solutions of ionic. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. the electrolysis of sodium chloride solution. Why Are Carbon Electrodes Used In Electrolysis.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Why Are Carbon Electrodes Used In Electrolysis the electrolysis of sodium chloride solution using carbon electrodes. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. Sodium is well above hydrogen in the electrochemical series and so, using the. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly.. Why Are Carbon Electrodes Used In Electrolysis.
From wiener.me
In The Electrolysis Of CuSO4 Using Copper Cathode And, 47 OFF Why Are Carbon Electrodes Used In Electrolysis introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. Sodium is well above hydrogen in the electrochemical series and so, using the. they just provide a surface for ions to gain or lose electrons so they form products. carbon electrodes are used in electrolysis due to their competence as a conductor and. Why Are Carbon Electrodes Used In Electrolysis.
From byjus.com
Explain the electrolysis of copper sulphate solution with the help of a Why Are Carbon Electrodes Used In Electrolysis carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. This page looks in detail at the electrolysis of solutions of ionic. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. this article here indicates that carbon/graphite electrodes are attacked both. Why Are Carbon Electrodes Used In Electrolysis.
From dxohpueyt.blob.core.windows.net
Surface Area Of Electrodes In Electrolysis at Diane Ashton blog Why Are Carbon Electrodes Used In Electrolysis carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. Sodium is well above hydrogen in the electrochemical series and so, using the. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. they just provide a surface. Why Are Carbon Electrodes Used In Electrolysis.
From www.mbrashem.com
Why Are Graphite Electrodes Used in Electrolysis? M. Brashem, Inc Why Are Carbon Electrodes Used In Electrolysis the electrolysis of sodium chloride solution using carbon electrodes. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. This page looks in detail at the electrolysis of solutions of ionic. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. they just provide a surface for. Why Are Carbon Electrodes Used In Electrolysis.
From www.energy.gov
Hydrogen Production Electrolysis Department of Energy Why Are Carbon Electrodes Used In Electrolysis the electrolysis of aqueous solutions of ionic compounds using inert electrodes. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Graphite (a form of carbon) and platinum. carbon electrodes have the bulk amount. Why Are Carbon Electrodes Used In Electrolysis.
From jinsuncarbon.com
Why are graphite electrodes used in electrolysis? Why Are Carbon Electrodes Used In Electrolysis carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the electrolysis of aqueous solutions of ionic compounds using inert electrodes. Sodium is well above hydrogen in the electrochemical series and so,. Why Are Carbon Electrodes Used In Electrolysis.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? Why Are Carbon Electrodes Used In Electrolysis the electrolysis of aqueous solutions of ionic compounds using inert electrodes. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. they just provide a surface for ions to gain or lose electrons so they form products. This page looks in detail at the electrolysis of. Why Are Carbon Electrodes Used In Electrolysis.
From courses.lumenlearning.com
Predicting the Products of Electrolysis Introduction to Chemistry Why Are Carbon Electrodes Used In Electrolysis Graphite (a form of carbon) and platinum. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen. Why Are Carbon Electrodes Used In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation ID297961 Why Are Carbon Electrodes Used In Electrolysis they just provide a surface for ions to gain or lose electrons so they form products. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. Sodium is well above hydrogen in the electrochemical series and so, using the. the electrolysis of sodium chloride solution using carbon electrodes. . Why Are Carbon Electrodes Used In Electrolysis.
From www.snexplores.org
Explainer What is an electrode? Why Are Carbon Electrodes Used In Electrolysis this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. the electrolysis of aqueous solutions of ionic compounds using inert electrodes. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. the electrolysis of sodium chloride solution using carbon electrodes. in any electrochemical cell (electrolytic or. Why Are Carbon Electrodes Used In Electrolysis.
From www.microteknik.com
Electrolysisapparatus Why Are Carbon Electrodes Used In Electrolysis carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. Sodium is well above hydrogen in the electrochemical series and so, using the. the electrolysis of sodium chloride solution using carbon electrodes. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. this article. Why Are Carbon Electrodes Used In Electrolysis.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Why Are Carbon Electrodes Used In Electrolysis the electrolysis of sodium chloride solution using carbon electrodes. they just provide a surface for ions to gain or lose electrons so they form products. Graphite (a form of carbon) and platinum. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. the electrolysis of aqueous solutions of ionic compounds using. Why Are Carbon Electrodes Used In Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Why Are Carbon Electrodes Used In Electrolysis Sodium is well above hydrogen in the electrochemical series and so, using the. they just provide a surface for ions to gain or lose electrons so they form products. Graphite (a form of carbon) and platinum. the electrolysis of sodium chloride solution using carbon electrodes. carbon electrodes have the bulk amount of free electrons that are free. Why Are Carbon Electrodes Used In Electrolysis.
From question.pandai.org
Electrolytic Cell Why Are Carbon Electrodes Used In Electrolysis Sodium is well above hydrogen in the electrochemical series and so, using the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. this article here indicates that carbon/graphite electrodes are attacked both chemically (oxygen gets converted to. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity. Why Are Carbon Electrodes Used In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Why Are Carbon Electrodes Used In Electrolysis Graphite (a form of carbon) and platinum. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the ability as a conductor. carbon electrodes are used in electrolysis due to their competence as a. Why Are Carbon Electrodes Used In Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Why Are Carbon Electrodes Used In Electrolysis Graphite (a form of carbon) and platinum. carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. This page looks in detail at the electrolysis of solutions of ionic. Sodium is well above hydrogen in the electrochemical series and so, using the. carbon electrodes have the bulk amount of free. Why Are Carbon Electrodes Used In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Why Are Carbon Electrodes Used In Electrolysis Sodium is well above hydrogen in the electrochemical series and so, using the. introducing carbon materials with different microstructures and morphologies, because the electronic conductivity is strongly. This page looks in detail at the electrolysis of solutions of ionic. they just provide a surface for ions to gain or lose electrons so they form products. carbon electrodes. Why Are Carbon Electrodes Used In Electrolysis.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation Why Are Carbon Electrodes Used In Electrolysis carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Graphite (a form of carbon) and platinum. the electrolysis of sodium chloride solution using carbon electrodes. introducing carbon materials with different. Why Are Carbon Electrodes Used In Electrolysis.
From www.amazon.in
SWISSO 5 X Pure Graphite Electrode 15mm High Purity Graphite Rod 99.99 Why Are Carbon Electrodes Used In Electrolysis they just provide a surface for ions to gain or lose electrons so they form products. Sodium is well above hydrogen in the electrochemical series and so, using the. This page looks in detail at the electrolysis of solutions of ionic. carbon electrodes have the bulk amount of free electrons that are free for transfer and have the. Why Are Carbon Electrodes Used In Electrolysis.