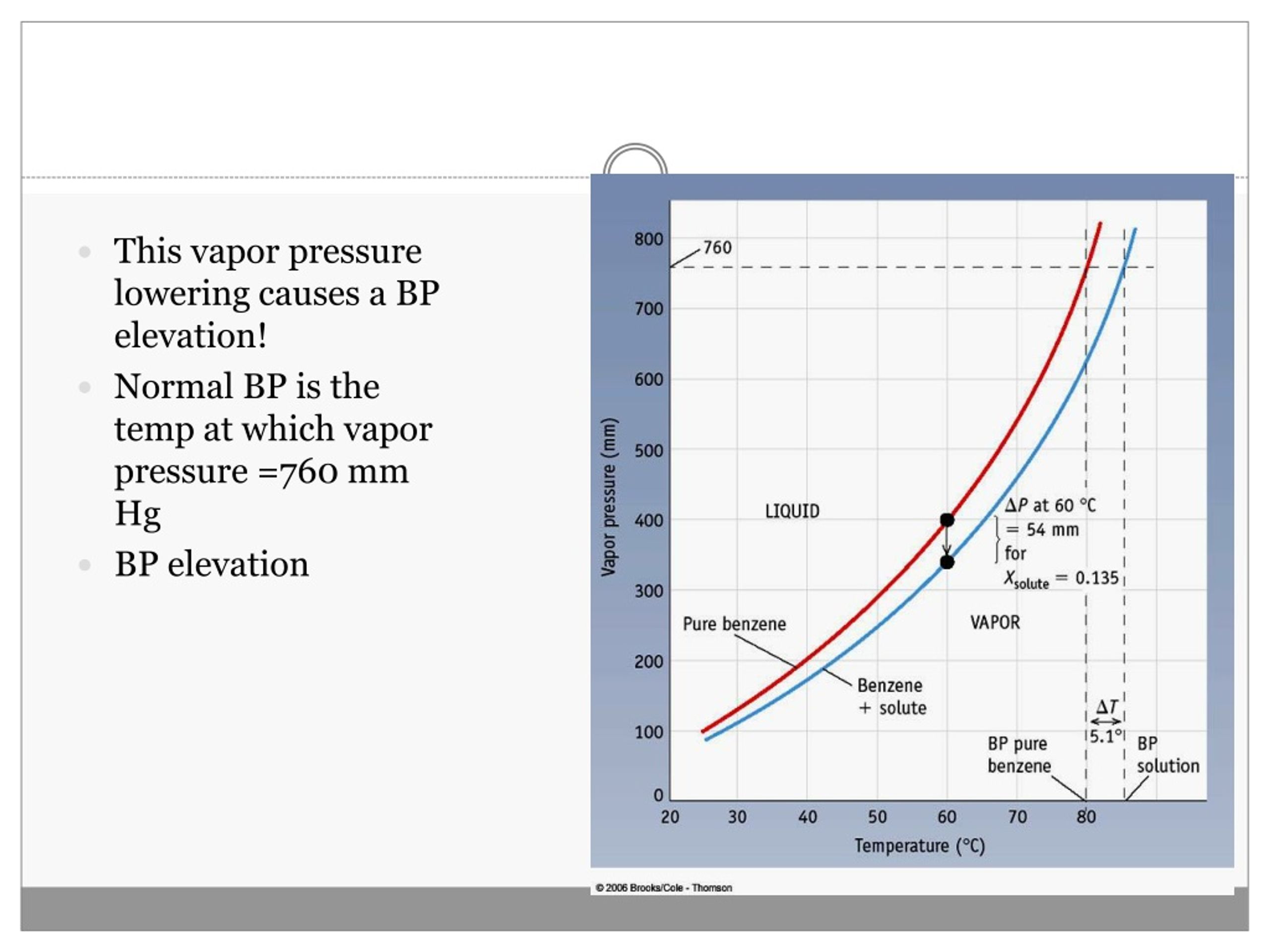
What Causes Vapor Pressure Lowering . The molecules on a liquid 's surface experience. The lowering of the vapor. The vapor pressure lowering is directly. Vapor pressure lowering is a colligative property of solutions. The nonlinear increase in vapor pressure with. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. So, first, what is vapor pressure? To understand vapor pressure lowering, consider surface tension. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. Why does vapor pressure lower? The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent.
from www.slideserve.com
The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. So, first, what is vapor pressure? The nonlinear increase in vapor pressure with. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. The vapor pressure lowering is directly. To understand vapor pressure lowering, consider surface tension. The lowering of the vapor.
PPT Chapter 12 PowerPoint Presentation, free download ID9100347
What Causes Vapor Pressure Lowering When the molecule escape the liquid there. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. So, first, what is vapor pressure? The molecules on a liquid 's surface experience. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. Vapor pressure lowering is a colligative property of solutions. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The lowering of the vapor. The nonlinear increase in vapor pressure with. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given. Why does vapor pressure lower? The vapor pressure lowering is directly. To understand vapor pressure lowering, consider surface tension. When the molecule escape the liquid there.
From chemyam.blogspot.com
COLLIGATIVE PROPERTIES (Relative lowering of vapour pressure) What Causes Vapor Pressure Lowering To understand vapor pressure lowering, consider surface tension. Vapor pressure lowering is a colligative property of solutions. So, first, what is vapor pressure? The nonlinear increase in vapor pressure with. The lowering of the vapor. Why does vapor pressure lower? The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given.. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3588530 What Causes Vapor Pressure Lowering Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download What Causes Vapor Pressure Lowering Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. When the molecule escape the liquid there. Vapor pressure lowering is a colligative property of solutions. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given. The. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation ID5410728 What Causes Vapor Pressure Lowering The molecules on a liquid 's surface experience. When the molecule escape the liquid there. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. The. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download What Causes Vapor Pressure Lowering Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. Vapor pressure lowering is a colligative property of solutions. The molecules on a liquid 's surface experience. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as. What Causes Vapor Pressure Lowering.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Causes Vapor Pressure Lowering The lowering of the vapor. The nonlinear increase in vapor pressure with. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. So, first, what is vapor pressure? When the molecule escape the liquid there. To understand vapor pressure lowering, consider surface tension. The pressure exerted by the gas in. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID9100347 What Causes Vapor Pressure Lowering Why does vapor pressure lower? Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. The vapor pressure lowering is directly. When the molecule escape the liquid there. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. The nonlinear. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT SOLUTIONS PowerPoint Presentation, free download ID1598971 What Causes Vapor Pressure Lowering Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. The vapor pressure lowering is directly. To understand vapor pressure lowering, consider surface tension. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Recall that when. What Causes Vapor Pressure Lowering.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Causes Vapor Pressure Lowering The vapor pressure lowering is directly. The molecules on a liquid 's surface experience. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. So, first, what is. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download What Causes Vapor Pressure Lowering The molecules on a liquid 's surface experience. Why does vapor pressure lower? Vapor pressure lowering is a colligative property of solutions. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid.. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Molality and Mole Fraction PowerPoint Presentation ID259070 What Causes Vapor Pressure Lowering The lowering of the vapor. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The nonlinear increase in vapor pressure with. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. Since the solute particles do not evaporate,. What Causes Vapor Pressure Lowering.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Causes Vapor Pressure Lowering To understand vapor pressure lowering, consider surface tension. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. So, first, what is vapor pressure? The lowering of the vapor. The molecules on a liquid 's surface experience. Vapor pressure lowering is a colligative property of solutions. The force that. What Causes Vapor Pressure Lowering.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Causes Vapor Pressure Lowering When the molecule escape the liquid there. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given. Recall that when a liquid evaporates in a closed container,. What Causes Vapor Pressure Lowering.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Causes Vapor Pressure Lowering Vapor pressure lowering is a colligative property of solutions. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. To understand vapor pressure lowering, consider surface tension. The molecules on a liquid 's surface experience. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2968601 What Causes Vapor Pressure Lowering Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. Why does vapor pressure lower? The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Vapor Pressure Lowering PowerPoint Presentation, free download What Causes Vapor Pressure Lowering The nonlinear increase in vapor pressure with. When the molecule escape the liquid there. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: Vapor pressure lowering is a colligative property of solutions. Why does vapor pressure lower? Recall that when a liquid evaporates in. What Causes Vapor Pressure Lowering.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Causes Vapor Pressure Lowering To understand vapor pressure lowering, consider surface tension. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. So, first, what is vapor pressure? The nonlinear increase in vapor. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID8714033 What Causes Vapor Pressure Lowering The lowering of the vapor. The nonlinear increase in vapor pressure with. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. Why does vapor pressure lower? When the. What Causes Vapor Pressure Lowering.
From www.ck12.org
VaporPressure Lowering (Raoult's Law) ΔP = X[B]P°[A] Example 1 What Causes Vapor Pressure Lowering Vapor pressure lowering is a colligative property of solutions. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. Why does vapor pressure lower? The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The force that. What Causes Vapor Pressure Lowering.
From www.youtube.com
Vapor Pressure Lowering worked Example YouTube What Causes Vapor Pressure Lowering Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. To understand vapor pressure lowering, consider surface tension. Why does vapor pressure lower? The molecules on a liquid 's surface experience. The lowering of the vapor. Recall that when a liquid evaporates in a closed container, an equilibrium is. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Chemistry B Colligative Properties PowerPoint Presentation, free What Causes Vapor Pressure Lowering The vapor pressure lowering is directly. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. Vapor pressure lowering is a colligative property of solutions. So, first, what is vapor pressure? When the molecule escape the liquid there. Why does vapor pressure lower? Since the solute particles do not evaporate, the vapor pressure. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Causes Vapor Pressure Lowering To understand vapor pressure lowering, consider surface tension. Why does vapor pressure lower? Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: Molecules. What Causes Vapor Pressure Lowering.
From www.youtube.com
Colligative Properties 1 Raoult's Law (Vapor Pressure Lowering) YouTube What Causes Vapor Pressure Lowering Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. The lowering of the vapor. To understand vapor pressure lowering, consider surface tension. So, first, what. What Causes Vapor Pressure Lowering.
From chemistnotes.com
Vapor Pressure, Lowering of Vapor Pressure, Definition, Equation What Causes Vapor Pressure Lowering The nonlinear increase in vapor pressure with. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. So, first, what is vapor pressure? Vapor pressure lowering is a colligative. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2965441 What Causes Vapor Pressure Lowering The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. When the molecule escape the liquid there. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: Vapor pressure lowering is a colligative property of. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT AP Chemistry Notes PowerPoint Presentation, free download ID What Causes Vapor Pressure Lowering To understand vapor pressure lowering, consider surface tension. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. The nonlinear increase in. What Causes Vapor Pressure Lowering.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Causes Vapor Pressure Lowering Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. The nonlinear increase in vapor pressure with. When the molecule escape the liquid there. Why does vapor pressure. What Causes Vapor Pressure Lowering.
From www.youtube.com
VaporPressure Lowering (Raoult's Law) ΔP = XBP°A YouTube What Causes Vapor Pressure Lowering The lowering of the vapor. To understand vapor pressure lowering, consider surface tension. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. Vapor pressure lowering is a colligative property of solutions. When the molecule escape the liquid there. The vapor pressure of a solvent in a. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID What Causes Vapor Pressure Lowering To understand vapor pressure lowering, consider surface tension. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given. The nonlinear increase in vapor pressure with. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. The vapor pressure lowering is directly. The vapor. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download What Causes Vapor Pressure Lowering The molecules on a liquid 's surface experience. Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. Since the solute particles do not evaporate, the vapor pressure of the solution is lower than that of the pure solvent. When the molecule escape the liquid there. Recall. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT COLLIGATIVE PROPERTIES PowerPoint Presentation, free download What Causes Vapor Pressure Lowering The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Vapor pressure lowering is a colligative property of solutions. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given. The lowering of the vapor. To understand vapor pressure lowering, consider. What Causes Vapor Pressure Lowering.
From www.slideserve.com
PPT Raoult’s Law Vapor Pressure Lowering PowerPoint Presentation What Causes Vapor Pressure Lowering Molecules that can hydrogen bond, such as ethylene glycol, have a much lower equilibrium vapor pressure than those that cannot, such as octane. Vapor pressure lowering is a colligative property of solutions. The molecules on a liquid 's surface experience. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent.. What Causes Vapor Pressure Lowering.
From inspiritvr.com
Vapor Pressure Lowering Study Guide Inspirit What Causes Vapor Pressure Lowering The vapor pressure lowering is directly. Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. So, first, what is vapor pressure? Why does vapor pressure lower? The lowering of the vapor. Vapor pressure lowering is a colligative property of solutions. Molecules that can hydrogen bond, such as ethylene glycol, have a much. What Causes Vapor Pressure Lowering.
From www.youtube.com
Colligative properties Vapor pressure lowering YouTube What Causes Vapor Pressure Lowering The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Vapor pressure lowering is a colligative property of solutions. So, first, what is vapor pressure? The nonlinear increase in vapor pressure with. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at. What Causes Vapor Pressure Lowering.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Causes Vapor Pressure Lowering The vapor pressure lowering is directly. The force that is driving the solvent molecules to the vapour phase is the difference between the entropy of the liquid and the vapour phases: Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid. Since the solute particles do not evaporate, the vapor pressure of the. What Causes Vapor Pressure Lowering.