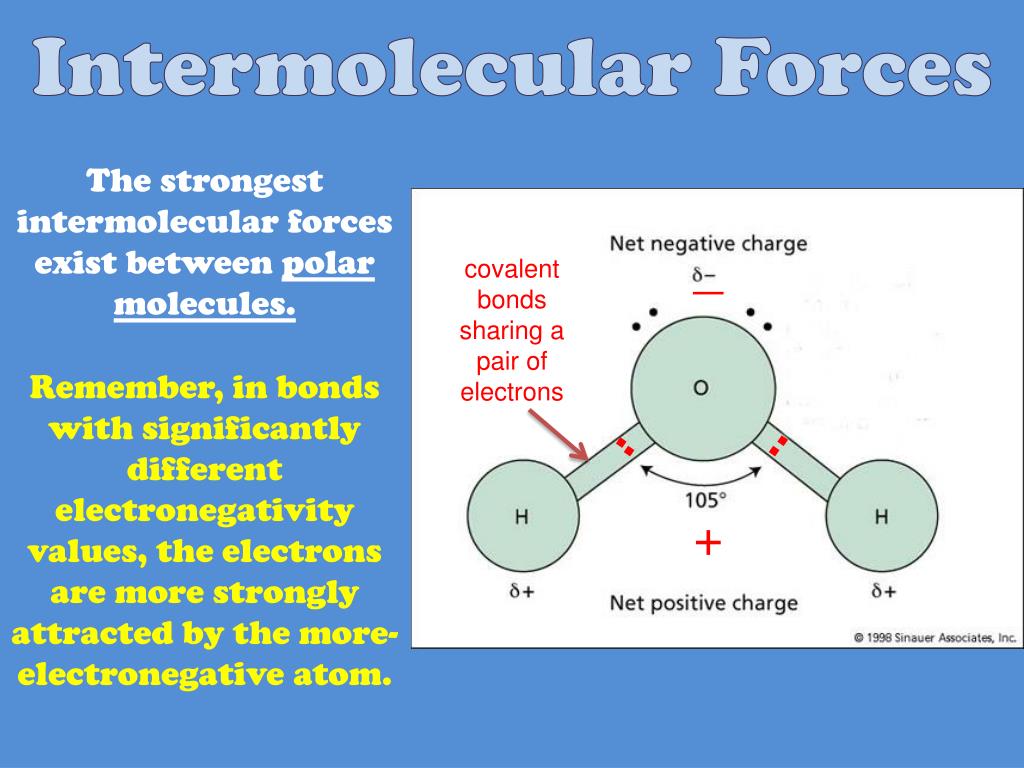
High Surface Tension Intermolecular Forces . Within the body of a liquid, molecules do not experience any force because. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Attraction between surface molecules in a liquid. As a result of this high surface tension, the surface of water. Water also has an exceptionally high heat of. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Attractive forces at the surface pull molecules inward causing surface. Water's high surface tension is due to the hydrogen bonding in water molecules. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces:
from www.slideserve.com
The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Attractive forces at the surface pull molecules inward causing surface. Attraction between surface molecules in a liquid. As a result of this high surface tension, the surface of water. Water also has an exceptionally high heat of. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Within the body of a liquid, molecules do not experience any force because. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Water's high surface tension is due to the hydrogen bonding in water molecules.
PPT Monday January 3 , 2011 PowerPoint Presentation, free download
High Surface Tension Intermolecular Forces Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. As a result of this high surface tension, the surface of water. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Water also has an exceptionally high heat of. Attractive forces at the surface pull molecules inward causing surface. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Attraction between surface molecules in a liquid. Within the body of a liquid, molecules do not experience any force because. Water's high surface tension is due to the hydrogen bonding in water molecules. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules.
From slideplayer.com
Intermolecular forces ppt download High Surface Tension Intermolecular Forces Water also has an exceptionally high heat of. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface tension, the surface of water. Within the body of a liquid, molecules do not experience any force because. Attraction between surface molecules in a liquid. Attractive forces. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint High Surface Tension Intermolecular Forces Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Within the body of a liquid, molecules do not experience any force because. Attractive forces at the surface pull molecules inward causing surface.. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint High Surface Tension Intermolecular Forces Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Water's high surface tension is due to the hydrogen bonding in water molecules. Attraction between surface molecules in a liquid. Within the body of a liquid, molecules. High Surface Tension Intermolecular Forces.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) High Surface Tension Intermolecular Forces Water also has an exceptionally high heat of. Within the body of a liquid, molecules do not experience any force because. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Water's high surface tension is due to the hydrogen bonding in water molecules. Intermolecular forces like hydrogen bonding and van der waals. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint High Surface Tension Intermolecular Forces As a result of this high surface tension, the surface of water. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Within the body of a liquid, molecules do not experience any force because. The core principle is that the stronger the imfs in the sample of molecules, the more strongly. High Surface Tension Intermolecular Forces.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts High Surface Tension Intermolecular Forces Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Attraction between surface molecules in a liquid. Within the body of a liquid, molecules do not experience any force because. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Water's high surface tension is due to the hydrogen. High Surface Tension Intermolecular Forces.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog High Surface Tension Intermolecular Forces Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Within the body of a liquid, molecules do not experience any force because. The core principle is that the stronger the imfs in the sample of molecules, the. High Surface Tension Intermolecular Forces.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity High Surface Tension Intermolecular Forces Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Water also has an exceptionally high heat of. Attractive forces at the surface pull molecules inward causing surface. Attraction between surface molecules in a liquid. The core principle is that the stronger the imfs in the sample of molecules, the more strongly. High Surface Tension Intermolecular Forces.
From www.numerade.com
SOLVED What causes surface tension in liquids? Name a substance that High Surface Tension Intermolecular Forces Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Attraction between surface molecules in a liquid. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Water also. High Surface Tension Intermolecular Forces.
From lessonschoolsimplistic.z5.web.core.windows.net
Characteristics Of Surface Tension High Surface Tension Intermolecular Forces The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. As a result of this high surface tension, the surface of water. Water also has an exceptionally high heat of. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Within the body. High Surface Tension Intermolecular Forces.
From www.learnatnoon.com
What is Surface Tension in Physics? High Surface Tension Intermolecular Forces Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. As a result of this high surface tension, the surface of water. Within the body of a liquid, molecules do not experience any force because. Attractive forces. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 High Surface Tension Intermolecular Forces Attraction between surface molecules in a liquid. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Within the body of a liquid, molecules do not experience any force because. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. As a result of this high surface tension,. High Surface Tension Intermolecular Forces.
From www.youtube.com
Intermolecular Forces Surface Tension, Vapor Pressure YouTube High Surface Tension Intermolecular Forces Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Attractive forces at the surface pull molecules inward causing surface. As a result of this high surface tension, the surface of water. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Attraction between surface molecules in a. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint High Surface Tension Intermolecular Forces Attraction between surface molecules in a liquid. Water's high surface tension is due to the hydrogen bonding in water molecules. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: As a result of. High Surface Tension Intermolecular Forces.
From www.studypool.com
SOLUTION Intermolecular forces and surface tension docx Studypool High Surface Tension Intermolecular Forces Water also has an exceptionally high heat of. Water's high surface tension is due to the hydrogen bonding in water molecules. Attractive forces at the surface pull molecules inward causing surface. Attraction between surface molecules in a liquid. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Among common liquids, water exhibits a distinctly high. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular forces Generalizing properties PowerPoint High Surface Tension Intermolecular Forces Within the body of a liquid, molecules do not experience any force because. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Note the correlation between the surface tension of a liquid and the strength of the. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint High Surface Tension Intermolecular Forces Water also has an exceptionally high heat of. Attractive forces at the surface pull molecules inward causing surface. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Within the body of a liquid, molecules do not experience any force because. Water's high surface tension is due to the hydrogen bonding in water molecules. Note the. High Surface Tension Intermolecular Forces.
From funsizephysics.com
What is Surface Tension? FunsizePhysics High Surface Tension Intermolecular Forces Attraction between surface molecules in a liquid. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Water also has an exceptionally high heat of. Attractive forces at the surface pull molecules inward causing surface. Water's high surface tension is due to the hydrogen bonding in water molecules. As a result of this high surface tension,. High Surface Tension Intermolecular Forces.
From slideplayer.com
Intermolecular Forces and ppt download High Surface Tension Intermolecular Forces Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Water also has an exceptionally high heat of. The core principle is that the stronger the imfs in the sample of molecules, the more strongly. High Surface Tension Intermolecular Forces.
From www.youtube.com
Surface tension and capillary action Intermolecular Forces meriSTEM High Surface Tension Intermolecular Forces Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: As a result of this high surface tension, the surface of water. Water's high surface tension is due to the hydrogen bonding in water molecules. Attraction between surface molecules in a liquid. Water also has an exceptionally high heat of. Among common liquids,. High Surface Tension Intermolecular Forces.
From slideplayer.com
Chemical Bonding and Nomenclature ppt download High Surface Tension Intermolecular Forces Attractive forces at the surface pull molecules inward causing surface. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Attraction between surface molecules in a liquid. Within the body of a liquid, molecules do not experience any force because. Among common liquids, water exhibits a distinctly high surface tension due to strong. High Surface Tension Intermolecular Forces.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru High Surface Tension Intermolecular Forces Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Attraction between surface molecules in a liquid. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. As a result. High Surface Tension Intermolecular Forces.
From www.studypool.com
SOLUTION Intermolecular forces and surface tension docx Studypool High Surface Tension Intermolecular Forces Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Within the body of a liquid, molecules do not experience any force because. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Attraction between surface molecules in a liquid. As a result of this. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Monday January 3 , 2011 PowerPoint Presentation, free download High Surface Tension Intermolecular Forces Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface tension, the surface of water. Note the correlation between the surface tension of a liquid and the strength of the intermolecular. High Surface Tension Intermolecular Forces.
From www.pinterest.com
Intermolecular Forces Graphic Organizer (dispersion, dipole, hydrogen High Surface Tension Intermolecular Forces The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Attraction between surface molecules in a liquid. Attractive forces. High Surface Tension Intermolecular Forces.
From favpng.com
Surface Tension Liquid Intermolecular Force Molecule Capillary Action High Surface Tension Intermolecular Forces Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Attraction between surface molecules in a liquid. Attractive forces at the surface pull molecules inward causing surface. Water's high surface tension is due to the. High Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download High Surface Tension Intermolecular Forces Water's high surface tension is due to the hydrogen bonding in water molecules. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Attraction between surface molecules in a liquid. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this. High Surface Tension Intermolecular Forces.
From www.studypool.com
SOLUTION Intermolecular forces and surface tension simulation Studypool High Surface Tension Intermolecular Forces Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface tension, the surface of water. Attraction between surface molecules in a liquid. The core principle is that the. High Surface Tension Intermolecular Forces.
From game-fans.ru
Лучшие материалы Что такое поверхностное натяжение воды High Surface Tension Intermolecular Forces Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Attraction between surface molecules in a liquid. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. As a result of. High Surface Tension Intermolecular Forces.
From www.chemedx.org
Measuring Surface Tension to Investigate Intermolecular Forces High Surface Tension Intermolecular Forces Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Within the body of a liquid, molecules do not experience any force because. As a result of this high surface tension, the surface of. High Surface Tension Intermolecular Forces.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL High Surface Tension Intermolecular Forces Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: As a result of this high surface tension, the surface of water. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Attractive forces at the surface pull molecules inward causing surface. Within the body of a liquid, molecules. High Surface Tension Intermolecular Forces.
From www.youtube.com
12.3 Intermolecular Forces in Action Surface Tension & Viscosity YouTube High Surface Tension Intermolecular Forces Attraction between surface molecules in a liquid. Attractive forces at the surface pull molecules inward causing surface. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Water's high surface tension is due to the hydrogen bonding. High Surface Tension Intermolecular Forces.
From worldwaterforum7.org
Why Does Water Have Such High Surface Tension? WWF7 Water Resources High Surface Tension Intermolecular Forces Within the body of a liquid, molecules do not experience any force because. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen. High Surface Tension Intermolecular Forces.
From www.vrogue.co
Ammonia Intermolecular Forces vrogue.co High Surface Tension Intermolecular Forces Water's high surface tension is due to the hydrogen bonding in water molecules. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. As a result of this high surface tension, the surface of water. Note the correlation between the surface tension of a liquid and the strength of the intermolecular forces: Among common liquids, water. High Surface Tension Intermolecular Forces.
From www.youtube.com
11.4 Intermolecular Forces in Action Surface Tension, Viscosity High Surface Tension Intermolecular Forces Water's high surface tension is due to the hydrogen bonding in water molecules. Intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together. Attractive forces at the surface pull molecules inward causing surface. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact,. Note the correlation between. High Surface Tension Intermolecular Forces.