What Is The Electrochemical Potential Equation . Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. The standard hydrogen electrode (she) is a reference. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Understand the concept of reference. Understand the concepts of standard electrode potential,.
from app.pandai.org
Understand the concept of reference. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Understand the concepts of standard electrode potential,. The standard hydrogen electrode (she) is a reference. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials.
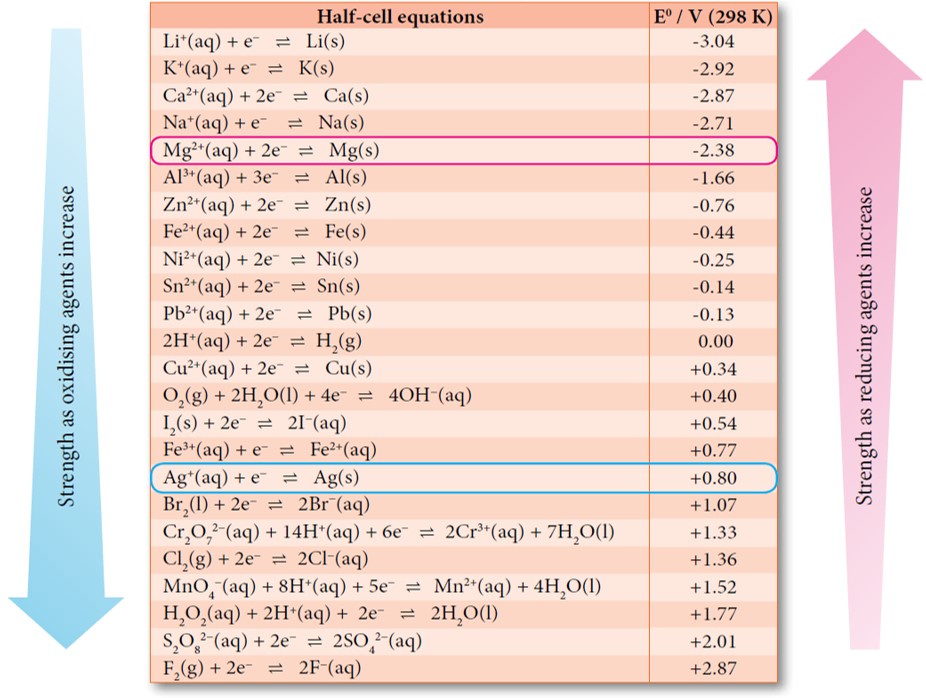
Determine oxidising agent and reducing agent based on value of standard
What Is The Electrochemical Potential Equation The standard hydrogen electrode (she) is a reference. Understand the concepts of standard electrode potential,. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Understand the concept of reference. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. The standard hydrogen electrode (she) is a reference. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 What Is The Electrochemical Potential Equation The standard hydrogen electrode (she) is a reference. Understand the concepts of standard electrode potential,. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants. What Is The Electrochemical Potential Equation.
From mrschimomot.blogspot.com
Gibbs Free Energy Formula In Electrochemistry Mrschimomot What Is The Electrochemical Potential Equation Understand the concepts of standard electrode potential,. The standard hydrogen electrode (she) is a reference. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Learn how to use standard electrode potentials to predict the direction and magnitude. What Is The Electrochemical Potential Equation.
From openbooks.lib.msu.edu
Membrane Potential Foundations of Neuroscience What Is The Electrochemical Potential Equation Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. The standard hydrogen electrode (she) is a reference. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products.. What Is The Electrochemical Potential Equation.
From scienceinfo.com
The Nernst Equation Derivation, Application, and Limitations What Is The Electrochemical Potential Equation At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. The standard hydrogen electrode (she) is a reference. Learn. What Is The Electrochemical Potential Equation.
From www.youtube.com
Using Standard Electrode Potentials YouTube What Is The Electrochemical Potential Equation Understand the concept of reference. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum. What Is The Electrochemical Potential Equation.
From www.nagwa.com
Question Video Identifying Conditions for Measuring Standard Electrode What Is The Electrochemical Potential Equation The standard hydrogen electrode (she) is a reference. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Understand the concepts of standard electrode potential,. At equilibrium, the electrochemical potential of any given species must be the same. What Is The Electrochemical Potential Equation.
From sites.google.com
Electrochemistry engineering chemistry What Is The Electrochemical Potential Equation The standard hydrogen electrode (she) is a reference. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Understand the concepts of standard electrode potential,. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants. What Is The Electrochemical Potential Equation.
From www.youtube.com
CLASS 12 JEE & NEET ELECTROCHEMISTRY NERNST EQUATION & ELECTRODE What Is The Electrochemical Potential Equation Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. The standard hydrogen electrode (she) is a reference. Understand the concepts of standard electrode potential,. Understand the concept of reference. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Learn the definition, measurement and applications of. What Is The Electrochemical Potential Equation.
From classnotes.org.in
Electrochemical Series Chemistry, Class 12, Electro Chemistry What Is The Electrochemical Potential Equation Understand the concept of reference. The standard hydrogen electrode (she) is a reference. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must. What Is The Electrochemical Potential Equation.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard What Is The Electrochemical Potential Equation At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Understand the concepts of standard electrode potential,. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how. What Is The Electrochemical Potential Equation.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch What Is The Electrochemical Potential Equation Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. The standard hydrogen electrode (she) is a reference. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Learn how to compare. What Is The Electrochemical Potential Equation.
From app.pandai.org
Determine oxidising agent and reducing agent based on value of standard What Is The Electrochemical Potential Equation Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Understand the concept of reference. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Understand the concepts of standard electrode potential,.. What Is The Electrochemical Potential Equation.
From www.youtube.com
What is the Cell Potential of the electrochemical cell in Which the What Is The Electrochemical Potential Equation Understand the concept of reference. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. The standard hydrogen. What Is The Electrochemical Potential Equation.
From 2012books.lardbucket.org
Electrochemistry What Is The Electrochemical Potential Equation Understand the concept of reference. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Understand the concepts of standard electrode potential,. The. What Is The Electrochemical Potential Equation.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts What Is The Electrochemical Potential Equation At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. The standard hydrogen electrode (she) is a reference. Understand. What Is The Electrochemical Potential Equation.
From www.youtube.com
calculation of half cell potential electrochemistry 2 class 12 What Is The Electrochemical Potential Equation Understand the concept of reference. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Understand the concepts of standard electrode potential,. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions.. What Is The Electrochemical Potential Equation.
From www.youtube.com
Cell Potential Problems Electrochemistry YouTube What Is The Electrochemical Potential Equation At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how to use standard electrode potentials to predict. What Is The Electrochemical Potential Equation.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent What Is The Electrochemical Potential Equation Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn how to compare the reactivities of metals using. What Is The Electrochemical Potential Equation.
From saylordotorg.github.io
Electrochemistry What Is The Electrochemical Potential Equation Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of. What Is The Electrochemical Potential Equation.
From www.pinterest.com
Electrochemistry Electrochemistry, Chemistry experiments, Chemistry What Is The Electrochemical Potential Equation Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Understand the concepts of standard electrode potential,. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. At equilibrium, the electrochemical potential. What Is The Electrochemical Potential Equation.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses What Is The Electrochemical Potential Equation Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Understand the concepts of standard electrode potential,. Understand the concept of reference. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions.. What Is The Electrochemical Potential Equation.
From mrschimomot.blogspot.com
Gibbs Free Energy Formula In Electrochemistry Mrschimomot What Is The Electrochemical Potential Equation Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Understand the concepts of standard electrode potential,. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. The standard hydrogen electrode (she) is a reference. At equilibrium, the electrochemical potential of any given species must be the same throughout. What Is The Electrochemical Potential Equation.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 What Is The Electrochemical Potential Equation Understand the concepts of standard electrode potential,. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Understand the concept of reference.. What Is The Electrochemical Potential Equation.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts What Is The Electrochemical Potential Equation Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. At equilibrium, the electrochemical potential of any given species must be the same. What Is The Electrochemical Potential Equation.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II What Is The Electrochemical Potential Equation Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants. What Is The Electrochemical Potential Equation.
From www.youtube.com
Emf of daniel cell from nernst equation(Electrochemistry part 28 for What Is The Electrochemical Potential Equation At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. The standard hydrogen electrode (she) is a reference. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions.. What Is The Electrochemical Potential Equation.
From www.youtube.com
Difference between Oxidation potential and Reduction potential What Is The Electrochemical Potential Equation The standard hydrogen electrode (she) is a reference. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Understand the concept of reference. Learn how to compare the reactivities of metals using simple equilibria. What Is The Electrochemical Potential Equation.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE What Is The Electrochemical Potential Equation The standard hydrogen electrode (she) is a reference. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Understand the concepts of standard electrode potential,. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Understand the concept of reference. At equilibrium, the electrochemical potential of any given species. What Is The Electrochemical Potential Equation.
From www.youtube.com
Introduction to Electroplating Electrochemistry YouTube What Is The Electrochemical Potential Equation Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants. What Is The Electrochemical Potential Equation.
From www.youtube.com
19.1 Calculating cell potential (HL) YouTube What Is The Electrochemical Potential Equation At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the electrochemical potentials of the reactants must equal those of the products. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn how to calculate and use electrochemical potential to. What Is The Electrochemical Potential Equation.
From www.slideserve.com
PPT Electrochemical Potential, Work, and Energy PowerPoint What Is The Electrochemical Potential Equation Understand the concepts of standard electrode potential,. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Understand the concept of reference. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions.. What Is The Electrochemical Potential Equation.
From www.chegg.com
Solved Electrochemical gradient is the driving force for ion What Is The Electrochemical Potential Equation Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Understand the concept of reference. The standard hydrogen electrode (she) is a. What Is The Electrochemical Potential Equation.
From www.youtube.com
Electrochemistry 9 Nernst Equation Find Electrode Potential or EMF What Is The Electrochemical Potential Equation Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn how to calculate and use electrochemical potential to predict the direction and magnitude of electrochemical reactions. The standard hydrogen electrode (she) is a reference. At equilibrium, the electrochemical potential. What Is The Electrochemical Potential Equation.
From socratic.org
In which of the following solution metallic ion can be displaced by What Is The Electrochemical Potential Equation Understand the concept of reference. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. At equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any chemical reaction, the sum of the. What Is The Electrochemical Potential Equation.
From www.slideserve.com
PPT Electrochemical Potential Non Standard Conditions Variations in What Is The Electrochemical Potential Equation Learn how to use standard electrode potentials to predict the direction and magnitude of electrochemical reactions. Understand the concepts of standard electrode potential,. Learn how to compare the reactivities of metals using simple equilibria and standard electrode potentials. Learn the definition, measurement and applications of electrochemical potential, the driving force for redox reactions. Learn how to calculate and use electrochemical. What Is The Electrochemical Potential Equation.