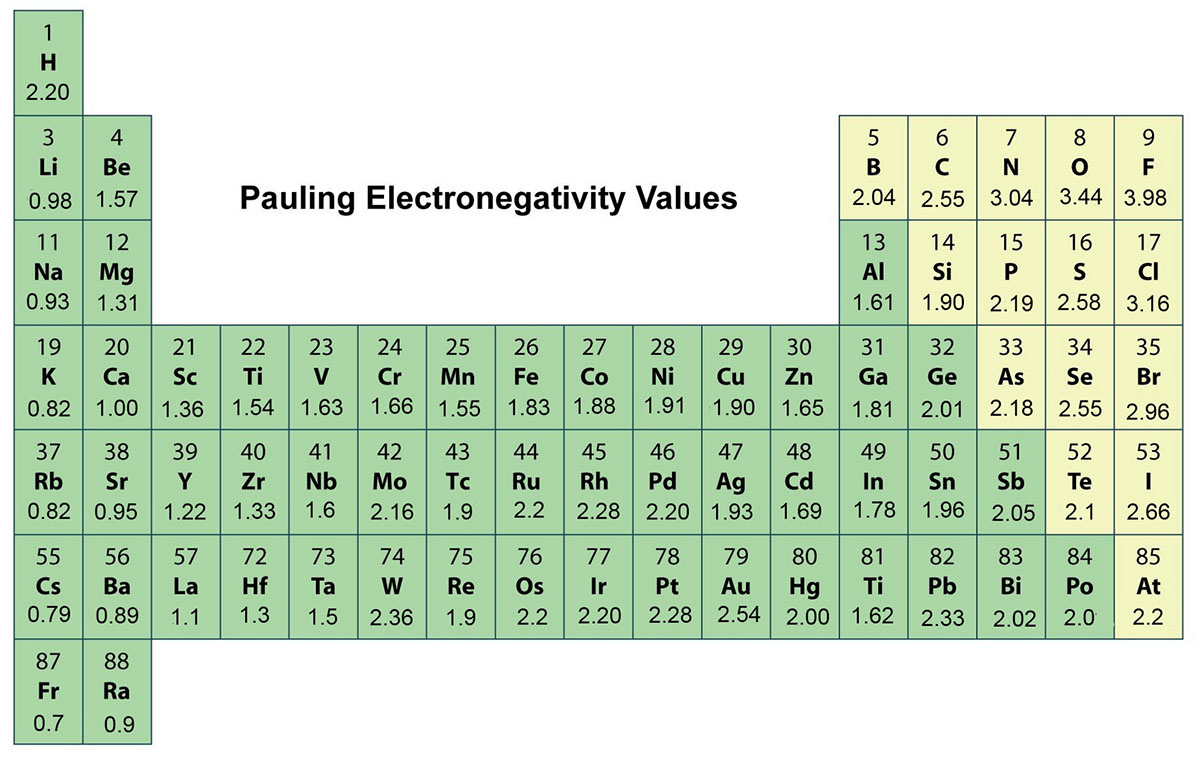
Electronegativity And Chlorine Bond . Given a pair of compounds, predict which would have a higher melting or boiling. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. Thus there is a direct correlation between. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. It is caused by the attractive electrostatic force. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. determine if a molecule is polar or nonpolar. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity.
from www.nanowerk.com
Thus there is a direct correlation between. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. It is caused by the attractive electrostatic force. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. Given a pair of compounds, predict which would have a higher melting or boiling. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. determine if a molecule is polar or nonpolar.
Electronegativity explained
Electronegativity And Chlorine Bond It is caused by the attractive electrostatic force. Thus there is a direct correlation between. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. Given a pair of compounds, predict which would have a higher melting or boiling. electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. determine if a molecule is polar or nonpolar. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. It is caused by the attractive electrostatic force. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Electronegativity And Chlorine Bond electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. It is caused by the attractive electrostatic force. electronegativity (χ) was defined as the ability of an atom in a molecule. Electronegativity And Chlorine Bond.
From www.youtube.com
Bond Type Using Electronegativity YouTube Electronegativity And Chlorine Bond Given a pair of compounds, predict which would have a higher melting or boiling. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. It is. Electronegativity And Chlorine Bond.
From chem.libretexts.org
8.4 Bond Polarity and Electronegativity Chemistry LibreTexts Electronegativity And Chlorine Bond electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. determine if a molecule is polar or nonpolar. It is caused by the attractive electrostatic force. electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. provided that. Electronegativity And Chlorine Bond.
From gioipnbxg.blob.core.windows.net
Element Chlorine Bonding at Jean Shinn blog Electronegativity And Chlorine Bond the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen. Electronegativity And Chlorine Bond.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent Electronegativity And Chlorine Bond whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen. Electronegativity And Chlorine Bond.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy visual Electronegativity And Chlorine Bond as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. Given a pair of compounds, predict which would have a higher melting or boiling. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. Thus there is a direct correlation. Electronegativity And Chlorine Bond.
From www.vedantu.com
Chemistry Chapter 4 Chemical Bonding Class 11 Notes Electronegativity And Chlorine Bond provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. determine if a molecule is polar or nonpolar. Thus there is a direct. Electronegativity And Chlorine Bond.
From chemdictionary.org
Electronegativity Definition And Examples Chemistry Dictionary Electronegativity And Chlorine Bond provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. whether a bond is nonpolar or polar covalent is determined by a property of the. Electronegativity And Chlorine Bond.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Electronegativity And Chlorine Bond electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. Thus there. Electronegativity And Chlorine Bond.
From www.sliderbase.com
Periodic Table of Electronegativities Electronegativity And Chlorine Bond electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. whether a bond is nonpolar or polar covalent is determined by. Electronegativity And Chlorine Bond.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Electronegativity And Chlorine Bond as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. It is caused by the attractive electrostatic force. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. whether a bond is nonpolar or polar. Electronegativity And Chlorine Bond.
From www.science-revision.co.uk
Electronegativity and polar bonds Electronegativity And Chlorine Bond electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl. Electronegativity And Chlorine Bond.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Electronegativity And Chlorine Bond as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. Given a pair of compounds, predict which would have a higher melting or boiling. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. electronegativity is the tendency of. Electronegativity And Chlorine Bond.
From sciencetrends.com
Electronegativity Chart Science Trends Electronegativity And Chlorine Bond electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. Given a pair of compounds, predict which would have a higher melting or boiling. as you go. Electronegativity And Chlorine Bond.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID9732450 Electronegativity And Chlorine Bond provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons. Electronegativity And Chlorine Bond.
From www.numerade.com
SOLVED Using the electronegativity chart, identify the bond type Electronegativity And Chlorine Bond the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. It is caused by the attractive electrostatic force. determine. Electronegativity And Chlorine Bond.
From www.slideserve.com
PPT Covalent Bonding and Nomenclature PowerPoint Presentation, free Electronegativity And Chlorine Bond Given a pair of compounds, predict which would have a higher melting or boiling. Thus there is a direct correlation between. It is caused by the attractive electrostatic force. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. the chlorine atom has a higher electronegativity than the hydrogen atom, so. Electronegativity And Chlorine Bond.
From chem.libretexts.org
8.7 Bond Polarity and Electronegativity (\(\chi\)) Chemistry LibreTexts Electronegativity And Chlorine Bond determine if a molecule is polar or nonpolar. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. whether a bond is nonpolar or polar covalent. Electronegativity And Chlorine Bond.
From slideplayer.com
CHEMICAL BONDING. ppt download Electronegativity And Chlorine Bond electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. provided that you understand what happens when electronegative atoms like fluorine. Electronegativity And Chlorine Bond.
From mavink.com
Element Electronegativity Chart Electronegativity And Chlorine Bond whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. determine if a molecule is polar or nonpolar. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. Thus there is a direct. Electronegativity And Chlorine Bond.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision Electronegativity And Chlorine Bond provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. determine if a molecule is polar or nonpolar. Given a pair of compounds, predict which would have a higher melting or boiling. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical. Electronegativity And Chlorine Bond.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Electronegativity And Chlorine Bond provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond.. Electronegativity And Chlorine Bond.
From giojcsdfs.blob.core.windows.net
Chlorine Ionic Bond Form at Janyce Gallegos blog Electronegativity And Chlorine Bond Thus there is a direct correlation between. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. electronegativity (χ) was defined as the ability of an atom in a. Electronegativity And Chlorine Bond.
From www.scribd.com
Electronegativity Questions PDF Chemical Bond Chlorine Electronegativity And Chlorine Bond Thus there is a direct correlation between. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. determine if a molecule is polar or nonpolar. It is caused by the attractive electrostatic force. electronegativity (χ) was defined as the ability of an atom in a molecule or an. Electronegativity And Chlorine Bond.
From www.numerade.com
SOLVED Consider the molecules below Classify each bond as an ionic Electronegativity And Chlorine Bond Thus there is a direct correlation between. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. electronegativity is the tendency of an atom to attract a pair of. Electronegativity And Chlorine Bond.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity And Chlorine Bond whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. determine if a molecule is polar or nonpolar. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. electronegativity (χ) was defined as the ability of an atom in a. Electronegativity And Chlorine Bond.
From slideplayer.com
State University of New York at Brockport ppt download Electronegativity And Chlorine Bond whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. determine if a molecule is polar or nonpolar. Given a pair of compounds, predict which would have a higher melting or boiling. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly. Electronegativity And Chlorine Bond.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Electronegativity And Chlorine Bond as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. electronegativity (χ) was defined as the ability of an atom in a molecule or an. Electronegativity And Chlorine Bond.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Electronegativity And Chlorine Bond Thus there is a direct correlation between. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. It is caused by the attractive electrostatic force. Given a pair of compounds, predict which. Electronegativity And Chlorine Bond.
From www.nanowerk.com
Electronegativity explained Electronegativity And Chlorine Bond Given a pair of compounds, predict which would have a higher melting or boiling. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Thus there is a direct correlation between. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. It. Electronegativity And Chlorine Bond.
From hxerxizou.blob.core.windows.net
Electronegativity Of Chlorine Is Higher Than Sulphur at Krista Electronegativity And Chlorine Bond determine if a molecule is polar or nonpolar. Thus there is a direct correlation between. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity.. Electronegativity And Chlorine Bond.
From thesciencecore.blogspot.com
covalent bond definition, properties and examples The Science Core Electronegativity And Chlorine Bond Given a pair of compounds, predict which would have a higher melting or boiling. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. the chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to. electronegativity. Electronegativity And Chlorine Bond.
From www.slideserve.com
PPT Bonding, Electronegativity , & Bond Shape PowerPoint Presentation Electronegativity And Chlorine Bond whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. determine if a molecule is polar or nonpolar. Thus there is a direct correlation between. electronegativity (χ) was. Electronegativity And Chlorine Bond.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity And Chlorine Bond It is caused by the attractive electrostatic force. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. Thus there is a direct correlation. Electronegativity And Chlorine Bond.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity And Chlorine Bond as you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the. Given a pair of compounds, predict which would have a higher melting or boiling. Thus there is a direct correlation between. electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract. Electronegativity And Chlorine Bond.