Rate Constant K Varies With Temperature By Equation . according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. L o g k (m i n − 1) = 5 − 2000 k t. The rate constant, however, does. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Rate constant k varies with temperature as given by equation:
from www.doubtnut.com
the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. L o g k (m i n − 1) = 5 − 2000 k t. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. Rate constant k varies with temperature as given by equation: The rate constant, however, does.
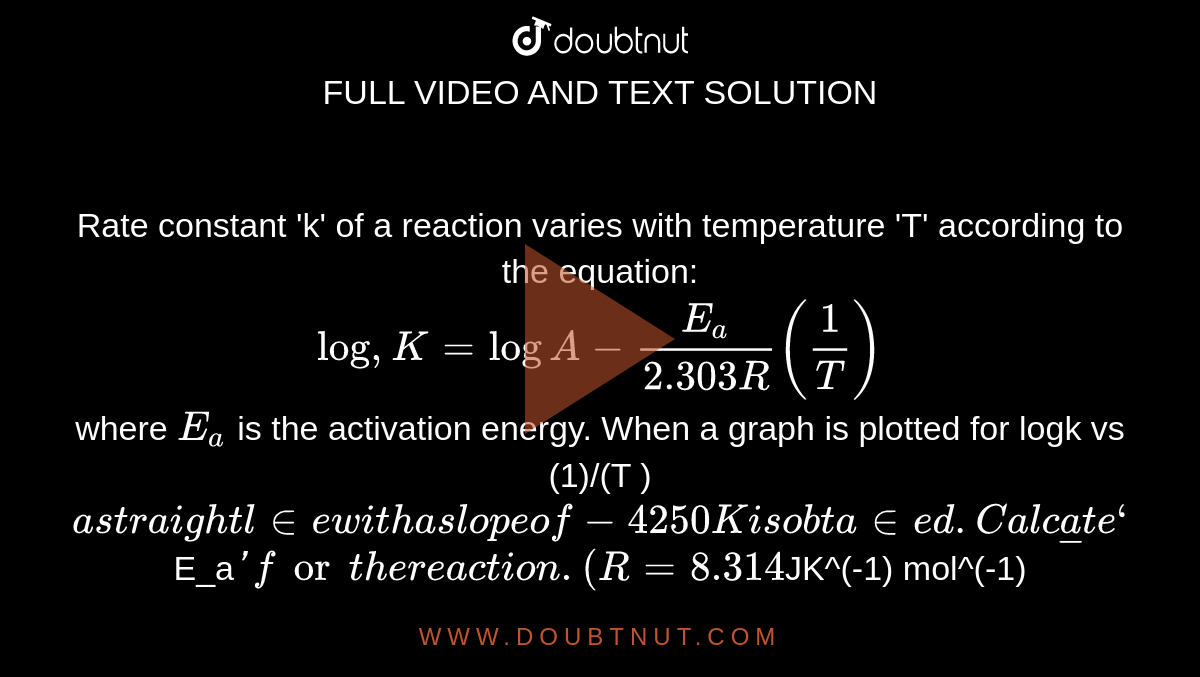
Rate constant 'k' of a reaction varies with temperature 'T' according
Rate Constant K Varies With Temperature By Equation Rate constant k varies with temperature as given by equation: Rate constant k varies with temperature as given by equation: L o g k (m i n − 1) = 5 − 2000 k t. The rate constant, however, does. according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration.
From fin3tutor.blogspot.com
How To Calculate Rate Constant With Temperature Rate Constant K Varies With Temperature By Equation the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. according to the arrhenius equation, k = a e − e. Rate Constant K Varies With Temperature By Equation.
From www.numerade.com
SOLVED Which one of the following given graphs represents the Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Rate constant k varies with temperature as given by equation: according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e. Rate Constant K Varies With Temperature By Equation.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K Varies With Temperature By Equation Rate constant k varies with temperature as given by equation: L o g k (m i n − 1) = 5 − 2000 k t. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. according to the arrhenius equation, k = a e −. Rate Constant K Varies With Temperature By Equation.
From www.chegg.com
Solved The rate constant k for a certain reaction is Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. L o g k (m i n − 1) = 5 − 2000 k t. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration.. Rate Constant K Varies With Temperature By Equation.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant K Varies With Temperature By Equation the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Rate constant k varies with temperature as given by equation: according. Rate Constant K Varies With Temperature By Equation.
From www.youtube.com
CHEM 201 Temperature Dependence of Rate Constant YouTube Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Rate constant k varies with temperature as given by equation: rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. according to the arrhenius. Rate Constant K Varies With Temperature By Equation.
From www.doubtnut.com
[Telugu] Rate constant K varies with temperature by equation log K("mi Rate Constant K Varies With Temperature By Equation according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. Rate constant k varies with temperature as given by equation: the rate constant k and the reaction orders m and n must be determined experimentally by observing how. Rate Constant K Varies With Temperature By Equation.
From haipernews.com
How To Calculate Equilibrium Constant At Different Temperatures Haiper Rate Constant K Varies With Temperature By Equation The rate constant, however, does. according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. L o g k (m i n − 1) = 5 − 2000 k t. the rate constant k and the reaction orders. Rate Constant K Varies With Temperature By Equation.
From www.youtube.com
Temperature and Arrhenius equation YouTube Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. L o g k (m i n − 1) = 5 − 2000 k t. The rate constant, however, does. rate = k[no][o 3] and is used to identify how the reaction rate (not the. Rate Constant K Varies With Temperature By Equation.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Rate Constant K Varies With Temperature By Equation L o g k (m i n − 1) = 5 − 2000 k t. The rate constant, however, does. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. the rate constant k is independent of the concentration of a, b, or c, but it does vary. Rate Constant K Varies With Temperature By Equation.
From www.youtube.com
How an Equilibrium Constant varies with Temperature Thermodynamics Rate Constant K Varies With Temperature By Equation the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. L o g k (m i n − 1) = 5 − 2000 k t.. Rate Constant K Varies With Temperature By Equation.
From www.slideserve.com
PPT Chapter 16 Rates and Mechanisms of Chemical Reactions Rate Constant K Varies With Temperature By Equation the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. L o g k (m. Rate Constant K Varies With Temperature By Equation.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube Rate Constant K Varies With Temperature By Equation L o g k (m i n − 1) = 5 − 2000 k t. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration.. Rate Constant K Varies With Temperature By Equation.
From answerhappy.com
+ The Arrhenius Equation The Arrhenius equation shows the relationship Rate Constant K Varies With Temperature By Equation L o g k (m i n − 1) = 5 − 2000 k t. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction.. Rate Constant K Varies With Temperature By Equation.
From askfilo.com
4. Which one of the following given graphs represents the variation of ra.. Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. Rate constant k varies with. Rate Constant K Varies With Temperature By Equation.
From askfilo.com
Plots showing the variation of the rate constant (k) with temperature (T).. Rate Constant K Varies With Temperature By Equation according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. Rate constant k varies with temperature as given by equation: L o g k (m i n − 1) = 5 − 2000 k t. the rate constant. Rate Constant K Varies With Temperature By Equation.
From www.researchgate.net
Variation of the rate constant k with temperature (a,b). • Chicory Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. L o g k (m. Rate Constant K Varies With Temperature By Equation.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant K Varies With Temperature By Equation Rate constant k varies with temperature as given by equation: The rate constant, however, does. according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. the rate constant k is independent of the concentration of a, b, or. Rate Constant K Varies With Temperature By Equation.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. The rate constant, however, does. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. according to the arrhenius equation, k. Rate Constant K Varies With Temperature By Equation.
From www.slideserve.com
PPT Reactions Rates and Temperature PowerPoint Presentation, free Rate Constant K Varies With Temperature By Equation Rate constant k varies with temperature as given by equation: The rate constant, however, does. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration.. Rate Constant K Varies With Temperature By Equation.
From www.slideserve.com
PPT Rate Laws PowerPoint Presentation, free download ID4905523 Rate Constant K Varies With Temperature By Equation rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. the rate constant k is independent of the concentration of a, b, or c,. Rate Constant K Varies With Temperature By Equation.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. rate = k[no][o 3] and is used to identify how the. Rate Constant K Varies With Temperature By Equation.
From www.numerade.com
SOLVED The rate constant of a reaction is measured at different Rate Constant K Varies With Temperature By Equation the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. The rate constant, however, does. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. L o g k (m i n. Rate Constant K Varies With Temperature By Equation.
From askfilo.com
The rate constant k of a reaction varies with temperature as lnk=2− T200 Rate Constant K Varies With Temperature By Equation L o g k (m i n − 1) = 5 − 2000 k t. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. The rate constant, however, does. Rate constant k varies with temperature as given by equation: the rate constant k and the reaction orders. Rate Constant K Varies With Temperature By Equation.
From www.solvedlib.com
Calculate three values for the rate constant; k, at r… SolvedLib Rate Constant K Varies With Temperature By Equation Rate constant k varies with temperature as given by equation: the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. L o. Rate Constant K Varies With Temperature By Equation.
From www.chegg.com
Solved The reaction rate constant, k, of the reaction A B Rate Constant K Varies With Temperature By Equation Rate constant k varies with temperature as given by equation: The rate constant, however, does. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration.. Rate Constant K Varies With Temperature By Equation.
From www.chegg.com
Solved The rate constant k for a certain reaction is Rate Constant K Varies With Temperature By Equation The rate constant, however, does. Rate constant k varies with temperature as given by equation: according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. the rate constant k is independent of the concentration of a, b, or. Rate Constant K Varies With Temperature By Equation.
From haipernews.com
How To Calculate Equilibrium Constant K Haiper Rate Constant K Varies With Temperature By Equation rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Rate constant k varies with temperature as given by equation: the rate constant k. Rate Constant K Varies With Temperature By Equation.
From kunduz.com
[ANSWERED] Rate constant K varies with temperature as given by equation Rate Constant K Varies With Temperature By Equation L o g k (m i n − 1) = 5 − 2000 k t. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate. Rate Constant K Varies With Temperature By Equation.
From www.doubtnut.com
Rate constant 'k' of a reaction varies with temperature 'T' according Rate Constant K Varies With Temperature By Equation the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. The rate constant, however, does. Rate constant k varies with temperature as. Rate Constant K Varies With Temperature By Equation.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K Varies With Temperature By Equation Rate constant k varies with temperature as given by equation: the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. L o g k (m i n − 1) = 5 − 2000 k t. the rate constant k and the reaction orders m and. Rate Constant K Varies With Temperature By Equation.
From www.chegg.com
Solved The temperature dependence of the reaction rate Rate Constant K Varies With Temperature By Equation according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. The rate constant, however, does. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction.. Rate Constant K Varies With Temperature By Equation.
From chart-studio.plotly.com
Rate constant in function of temperature scatter chart made by Ayoub Rate Constant K Varies With Temperature By Equation the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. according to the arrhenius equation, k = a e − e. Rate Constant K Varies With Temperature By Equation.
From www.numerade.com
SOLVEDThe rate constant (k) for a reaction was measured as a function Rate Constant K Varies With Temperature By Equation the rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. rate = k[no][o 3] and is used to identify how the reaction rate (not the rate constant) vares with concentration. L o g k (m i n − 1) = 5 − 2000 k t.. Rate Constant K Varies With Temperature By Equation.
From www.slideserve.com
PPT Reactions Rates and Temperature PowerPoint Presentation, free Rate Constant K Varies With Temperature By Equation according to the arrhenius equation, k = a e − e a / r t, as the temperature (t) increases, the exponential term, − e a / r t, becomes. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Rate constant k varies with. Rate Constant K Varies With Temperature By Equation.