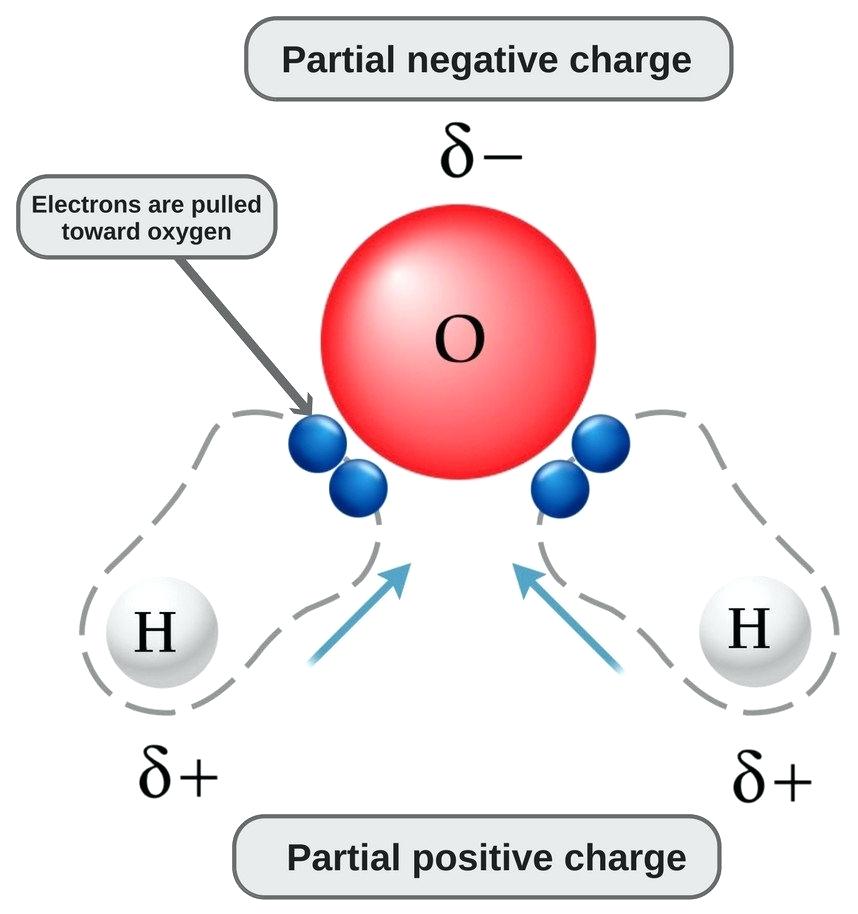
Water Molecules Dissolve Many Substances Because Of Its Polarity . Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Because of water's polarity, it is able to dissolve or dissociate many particles. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. And, water is called the. Because of water's polarity, it is able to dissolve or dissociate many particles. The process by which a. Oxygen has a slightly negative charge, while the two. Oxygen has a slightly negative charge, while the two.
from taylorsciencegeeks.weebly.com
The process by which a. Oxygen has a slightly negative charge, while the two. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Because of water's polarity, it is able to dissolve or dissociate many particles. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Because of water's polarity, it is able to dissolve or dissociate many particles. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Oxygen has a slightly negative charge, while the two.
The Water Molecule Science News
Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. The process by which a. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Oxygen has a slightly negative charge, while the two. And, water is called the. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Because of water's polarity, it is able to dissolve or dissociate many particles. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. Because of water's polarity, it is able to dissolve or dissociate many particles. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Oxygen has a slightly negative charge, while the two.
From lessonlibunidealism.z13.web.core.windows.net
Water As A Universal Solvent Notes Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a slightly negative charge, while the two. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. The process by which a. Water dissolves many biomolecules, because they are polar and. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From sciencenotes.org
Why Is Water Called the Universal Solvent? Water Molecules Dissolve Many Substances Because Of Its Polarity Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. The process by which a. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Oxygen has a slightly negative charge, while the two. Oxygen has a slightly negative charge, while. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From gotbooks.miracosta.edu
gotbooks.miracosta.edu/oceans Water Molecules Dissolve Many Substances Because Of Its Polarity The process by which a. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. And, water is called the. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the.. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary Water Molecules Dissolve Many Substances Because Of Its Polarity Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Because of water's polarity, it is able to dissolve or dissociate many particles. Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a slightly negative charge, while the two. The process by. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From mavink.com
Polar Water Molecule Diagram Water Molecules Dissolve Many Substances Because Of Its Polarity Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Because of water's polarity, it is able to dissolve or dissociate many particles. And, water is called the. The process by which a. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Oxygen has a slightly. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From taylorsciencegeeks.weebly.com
The Water Molecule Science News Water Molecules Dissolve Many Substances Because Of Its Polarity Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Because of water's polarity, it is able to dissolve or dissociate many particles.. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.science-revision.co.uk
Covalent bonding Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. The process by which a. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Special properties of water are its high heat capacity and heat of. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.pinterest.se
Polarity of Water Molecules infographic diagram showing its microscopic view along with crystal Water Molecules Dissolve Many Substances Because Of Its Polarity Oxygen has a slightly negative charge, while the two. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. The process by which a. And, water is called the. Oxygen has a slightly negative charge, while the two. Because of water's polarity,. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From socratic.org
What kinds of molecules are polar? + Example Water Molecules Dissolve Many Substances Because Of Its Polarity Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. And, water is called the. Oxygen has a slightly negative charge, while the two. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From slideplayer.com
Molecules & More Unit 3 Lecture ppt download Water Molecules Dissolve Many Substances Because Of Its Polarity Oxygen has a slightly negative charge, while the two. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. And, water is called the.. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From courses.lumenlearning.com
The Structure and Properties of Water Introduction to Chemistry Water Molecules Dissolve Many Substances Because Of Its Polarity Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Oxygen has a slightly negative charge, while the two. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Oxygen has a slightly negative charge, while the two. Water. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From exozucqop.blob.core.windows.net
Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding at Kevin Gilbert blog Water Molecules Dissolve Many Substances Because Of Its Polarity The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Oxygen has a slightly negative charge, while the two. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From slideplayer.com
Molecules & More Unit 3 Lecture ppt download Water Molecules Dissolve Many Substances Because Of Its Polarity Oxygen has a slightly negative charge, while the two. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Oxygen has a slightly negative charge, while the two. Water is capable of dissolving a variety of different substances, which is why it is such a good. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation ID5875746 Water Molecules Dissolve Many Substances Because Of Its Polarity The process by which a. Oxygen has a slightly negative charge, while the two. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Because of water's polarity, it is able to dissolve or dissociate many particles. And, water is called the. Water dissolves many biomolecules, because they are polar and. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT ELECTRONEGATIVITY POLAR BONDS MOLECULAR POLARITY PowerPoint Presentation ID4261659 Water Molecules Dissolve Many Substances Because Of Its Polarity Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. The process by which a. Oxygen has a slightly negative charge, while the two. Water is capable of dissolving a variety of different substances, which is why it is such a good. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Chemistry in Biology PowerPoint Presentation, free download ID2047099 Water Molecules Dissolve Many Substances Because Of Its Polarity Oxygen has a slightly negative charge, while the two. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. The process by which a. And, water is called the. Because of water's polarity, it is able to dissolve or dissociate many particles. Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a slightly. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic Bonding, Covalent Bonding Water Molecules Dissolve Many Substances Because Of Its Polarity The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. And, water is called the. Oxygen has a slightly negative charge, while the two.. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download ID1597821 Water Molecules Dissolve Many Substances Because Of Its Polarity Oxygen has a slightly negative charge, while the two. And, water is called the. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water dissolves many. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.mindomo.com
Science Mind Map Water Molecules Dissolve Many Substances Because Of Its Polarity Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. The process by which a. Water is capable of dissolving. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download ID9165094 Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID1926480 Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a slightly negative charge, while the two. Because of water's polarity, it is able to dissolve or dissociate many particles. The process by which a. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Special. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From slideplayer.com
Molecules & More Unit 3 Lecture ppt download Water Molecules Dissolve Many Substances Because Of Its Polarity And, water is called the. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Oxygen has a slightly negative. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From studylib.net
Polar and Nonpolar Molecules Water Molecules Dissolve Many Substances Because Of Its Polarity Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. Because of water's polarity, it is able to dissolve or. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From stock.adobe.com
the polar covalent bonds of water molecules (H2O) Stock Vector Adobe Stock Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Polar. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Water Chemistry PowerPoint Presentation, free download ID6898540 Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. The process by which a. And, water is called the. Oxygen has a slightly negative charge, while the two. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From saylordotorg.github.io
Aqueous Solutions Water Molecules Dissolve Many Substances Because Of Its Polarity Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and. The process by which a. Oxygen has a slightly negative charge, while the two. Because of water's polarity, it is able to dissolve or dissociate many particles. Polar molecules readily dissolve in water because the positive part of. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Chapter 2 Water PowerPoint Presentation, free download ID262198 Water Molecules Dissolve Many Substances Because Of Its Polarity Water dissolves many biomolecules, because they are polar and therefore hydrophilic. The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Oxygen has a slightly negative charge,. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From animalia-life.club
Properties Of Water Polarity Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a slightly negative charge, while the two. And, water is called the. Because of water's polarity, it is able to dissolve or dissociate many particles. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. The. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.britannica.com
polarity Definition & Examples Britannica Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Because of water's polarity, it is able to dissolve or dissociate many particles. Special properties of water are its high heat capacity and heat of vaporization, its ability. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.alamy.com
the polar covalent bonds of water molecules (H2O Stock Vector Image & Art Alamy Water Molecules Dissolve Many Substances Because Of Its Polarity The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. Oxygen has a slightly negative charge, while the two. And, water is called the. Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a slightly negative charge, while the two.. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID1977818 Water Molecules Dissolve Many Substances Because Of Its Polarity The net effect is a partial dipole, where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. And, water is called the. Oxygen has a slightly negative charge, while the two. Because of water's polarity, it is able to dissolve or dissociate many particles. Because of water's polarity, it is able to dissolve. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.acs.org
Lesson 5.1 Water is a Polar Molecule American Chemical Society Water Molecules Dissolve Many Substances Because Of Its Polarity Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Because of water's polarity, it is able to dissolve or dissociate many particles. The process by which a. Polar molecules readily dissolve in water because the positive part of the. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From slidetodoc.com
Properties of Water ETO Properties of Water Polar Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Because of water's polarity, it is able to dissolve or dissociate many particles. The process by which a. Oxygen has a slightly negative charge, while the two. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From slideplayer.com
Properties of Water. ppt download Water Molecules Dissolve Many Substances Because Of Its Polarity Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a slightly negative charge, while the two. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water dissolves many biomolecules, because they are polar and therefore hydrophilic. Because of water's polarity, it is able to. Water Molecules Dissolve Many Substances Because Of Its Polarity.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download ID9165094 Water Molecules Dissolve Many Substances Because Of Its Polarity Oxygen has a slightly negative charge, while the two. And, water is called the. Polar molecules readily dissolve in water because the positive part of the polar molecule is attracted to the oxygen atom, while the negative part is attracted to the. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar. Water Molecules Dissolve Many Substances Because Of Its Polarity.