Bromide Ion Nucleophile . it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. An example of the above is the reaction of. if the nucleophile being used is also a good base, it will prefer to take the proton.
from www.numerade.com
if the nucleophile being used is also a good base, it will prefer to take the proton. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. An example of the above is the reaction of. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond.
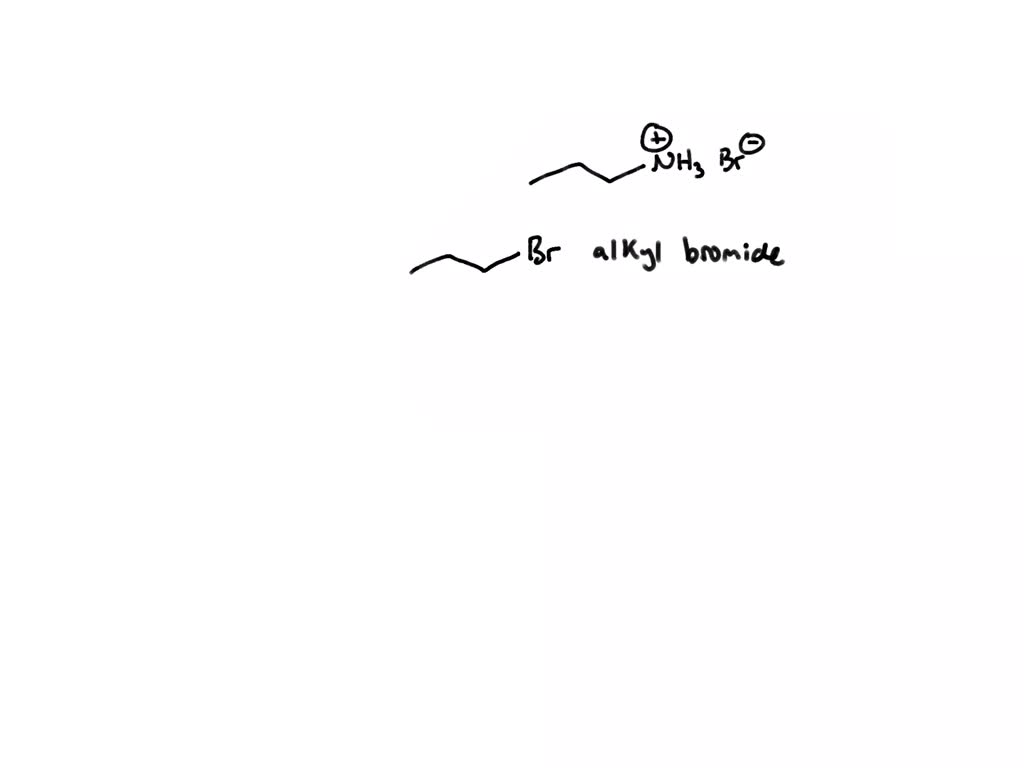
SOLVED 'Draw the structure of the alkyl bromide and the nucleophile
Bromide Ion Nucleophile a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. An example of the above is the reaction of. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. if the nucleophile being used is also a good base, it will prefer to take the proton. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? — Master Organic Chemistry Bromide Ion Nucleophile the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. An example of the above is the reaction of. it is a good leaving group because it. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED 2bromobutane reacts with sodium iodide in acetone. Why does Bromide Ion Nucleophile the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. if the nucleophile being used is also a good base, it will prefer to. Bromide Ion Nucleophile.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? — Master Organic Chemistry Bromide Ion Nucleophile recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. if the nucleophile being used is also a good base, it will prefer to take the proton. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron.. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED 1) Complete the curved arrow mechanism for the SN2 reaction Bromide Ion Nucleophile it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. if the nucleophile being used is also a good base, it will prefer to take the proton. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom. Bromide Ion Nucleophile.
From www.chegg.com
Solved Click on an atom that will react with the bromide ion Bromide Ion Nucleophile recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. if the nucleophile being used is also a good base, it will prefer to take the proton. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take. Bromide Ion Nucleophile.
From chem.libretexts.org
10.1 Nucleophilic Substitution Reactions of Alcohols Forming Alkyl Bromide Ion Nucleophile An example of the above is the reaction of. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. recall from section 7.3 that the basicity of atoms decreases as. Bromide Ion Nucleophile.
From www.masterorganicchemistry.com
The three types of nucleophiles you meet in organic chemistry — Master Bromide Ion Nucleophile An example of the above is the reaction of. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. a nucleophile is a reactant. Bromide Ion Nucleophile.
From www.reddit.com
How does the negatively charged bromide ion from the heterolytic Bromide Ion Nucleophile the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. a nucleophile is a reactant that provides a pair of electrons to form. Bromide Ion Nucleophile.
From www.youtube.com
Bromide Nucleophile vs Hydroxide Leaving Group YouTube Bromide Ion Nucleophile An example of the above is the reaction of. if the nucleophile being used is also a good base, it will prefer to take the proton. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. the bond between the carbon and the bromine then undergoes heterolytic. Bromide Ion Nucleophile.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromide Ion Nucleophile recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. An example of the above is the reaction of. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. in practical terms, this means that a hydroxide. Bromide Ion Nucleophile.
From www.science-revision.co.uk
Alkene addition reactions Bromide Ion Nucleophile the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. if the nucleophile being used is also a good base, it will prefer to. Bromide Ion Nucleophile.
From www.coursehero.com
[Solved] Identify the nucleophile in the Trityl Bromide and Ethanol Bromide Ion Nucleophile a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide. Bromide Ion Nucleophile.
From chem.libretexts.org
6.4 A Closer Look at the Nucleophilic Substitution Mechanism Bromide Ion Nucleophile recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. if the nucleophile being used is also a good base, it will prefer. Bromide Ion Nucleophile.
From www.doubtnut.com
Which is a better nucleophile, a bromide ion or an iodide ion? Bromide Ion Nucleophile if the nucleophile being used is also a good base, it will prefer to take the proton. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. recall from. Bromide Ion Nucleophile.
From www.numerade.com
SOLVEDMake a new bond between a nucleophile and an electrophile Bromide Ion Nucleophile An example of the above is the reaction of. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. it is a good leaving group because it leaves as bromide. Bromide Ion Nucleophile.
From www.masterorganicchemistry.com
The SN1 Mechanism — Master Organic Chemistry Bromide Ion Nucleophile a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED The reaction of isopentyl bromide with an anionic nucleophile Bromide Ion Nucleophile An example of the above is the reaction of. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. if the nucleophile being used is also a good base, it. Bromide Ion Nucleophile.
From www.chegg.com
Solved Identify the nucleophile in the Trityl Bromide and Bromide Ion Nucleophile An example of the above is the reaction of. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. recall. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED CH3 Brz CHzClz CH3 H3C Electrophilic addition of bromine Btz Bromide Ion Nucleophile a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. if the nucleophile being used is also a good base, it will prefer to take the proton. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (.. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED Consider the SN2 reaction between 2bromopropane and sodium Bromide Ion Nucleophile the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide. Bromide Ion Nucleophile.
From slideplayer.com
Halogenoalkanes and Reaction Pathways ppt download Bromide Ion Nucleophile the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. a nucleophile is a reactant that provides a pair of electrons to form. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED Draw the structure of the alkyl bromide and the nucleophile Bromide Ion Nucleophile a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. An example of the above is the reaction of. the bond between the carbon and the bromine. Bromide Ion Nucleophile.
From www.chegg.com
Solved Provide the alkyl bromide and nucleophile/base needed Bromide Ion Nucleophile the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. if the nucleophile being used is also a good base, it will prefer to take the proton.. Bromide Ion Nucleophile.
From www.chemistrysteps.com
SN2 Reaction Mechanism Bromide Ion Nucleophile recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide. Bromide Ion Nucleophile.
From www.masterorganicchemistry.com
Introduction to Nucleophilic Substitution Reactions Master Organic Bromide Ion Nucleophile in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. it is a good leaving group because it leaves as bromide ion, which is a weak base. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED Assuming that the ratedetermining step in the reaction of 1 Bromide Ion Nucleophile a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. An example of the above is the reaction of. if the nucleophile being used is also. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED 'Draw the structure of the alkyl bromide and the nucleophile Bromide Ion Nucleophile a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. if the nucleophile being used is also a good base, it will prefer to take the proton.. Bromide Ion Nucleophile.
From www.masterorganicchemistry.com
The Three Classes of Nucleophiles Master Organic Chemistry Bromide Ion Nucleophile if the nucleophile being used is also a good base, it will prefer to take the proton. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking. Bromide Ion Nucleophile.
From chemistrysteps.com
Reactions of Thiols Chemistry Steps Bromide Ion Nucleophile if the nucleophile being used is also a good base, it will prefer to take the proton. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide. Bromide Ion Nucleophile.
From www.numerade.com
SOLVED Toplcsi Biz CHZCIz Electrophilic addition of bromine, Biz t0 Bromide Ion Nucleophile if the nucleophile being used is also a good base, it will prefer to take the proton. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. a nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. in practical. Bromide Ion Nucleophile.
From www.youtube.com
Which is a better nucleophile, a bromide ion or an iodide ion? YouTube Bromide Ion Nucleophile An example of the above is the reaction of. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. if the nucleophile being used is also a good base, it will prefer to take the proton. a nucleophile is a reactant that provides. Bromide Ion Nucleophile.
From www.masterorganicchemistry.com
Addition of HBr to Alkenes Master Organic Chemistry Bromide Ion Nucleophile recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. the bond between the carbon and the bromine then undergoes heterolytic fission, with. Bromide Ion Nucleophile.
From www.chegg.com
Solved Electrophilic addition of bromine, Br2, to alkenes Bromide Ion Nucleophile if the nucleophile being used is also a good base, it will prefer to take the proton. the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic.. Bromide Ion Nucleophile.
From www.chegg.com
Solved Draw the structure of the alkyl bromide and the Bromide Ion Nucleophile it is a good leaving group because it leaves as bromide ion, which is a weak base and can take the electrons with it. An example of the above is the reaction of. in practical terms, this means that a hydroxide nucleophile will react in an s n 2 reaction with methyl bromide much faster (. the. Bromide Ion Nucleophile.
From pnghut.com
Electron Configuration Bromine Chemical Element Shell Bohr Model Bromide Ion Nucleophile the bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron. recall from section 7.3 that the basicity of atoms decreases as we move vertically down a column on the periodic. if the nucleophile being used is also a good base, it will prefer to take the proton.. Bromide Ion Nucleophile.