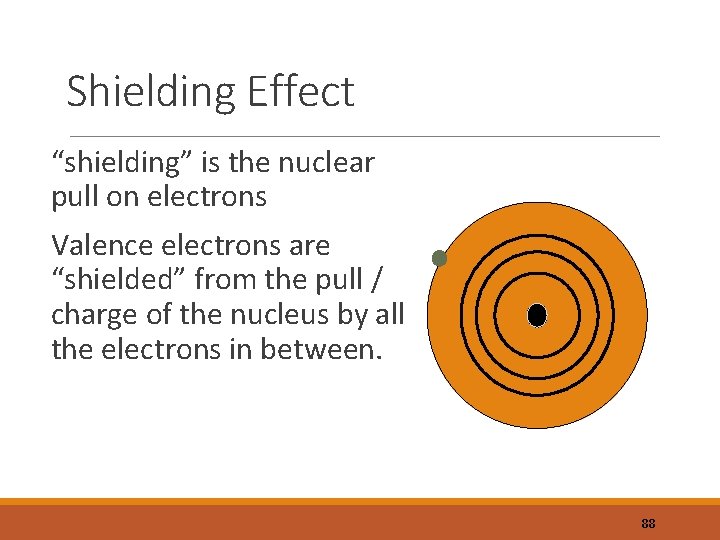
Electron Shielding Effect . electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Find out how core electrons repel outer electrons and. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). learn how electron shielding and penetration affect the physical and chemical properties of elements. Learn how it affects ionization. It manifests itself on macroscopic scales by. learn how electron shielding affects the potential energy and orbital energies of atoms and ions.
from slidetodoc.com
It manifests itself on macroscopic scales by. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Learn how it affects ionization. learn how electron shielding and penetration affect the physical and chemical properties of elements. Find out how core electrons repel outer electrons and. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. learn how electron shielding affects the potential energy and orbital energies of atoms and ions. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples.
Chapter 6 THE PERIODIC TABLE 1 Introduction Activity
Electron Shielding Effect electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Learn how it affects ionization. learn how electron shielding affects the potential energy and orbital energies of atoms and ions. learn how electron shielding and penetration affect the physical and chemical properties of elements. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. It manifests itself on macroscopic scales by. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). Find out how core electrons repel outer electrons and. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting Electron Shielding Effect Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Learn how it affects ionization. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Electrons in different orbitals have different wavefunctions and therefore different. Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2834450 Electron Shielding Effect It manifests itself on macroscopic scales by. Find out how core electrons repel outer electrons and. learn how electron shielding affects the potential energy and orbital energies of atoms and ions. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Effective nuclear charge, zeff, experienced by an electron is. Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2617702 Electron Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Find out how core electrons repel outer electrons and. Learn how it affects ionization. It manifests itself on macroscopic scales by. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m. Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation ID2834450 Electron Shielding Effect Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Learn how it affects ionization. electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical. Electron Shielding Effect.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding Electron Shielding Effect Learn how it affects ionization. It manifests itself on macroscopic scales by. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Find out how core electrons repel outer electrons and. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m. Electron Shielding Effect.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog Electron Shielding Effect Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Learn how it affects ionization. learn how electron shielding and penetration affect the physical and. Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Patterns PowerPoint Presentation, free download ID2877545 Electron Shielding Effect Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. learn how electron shielding and penetration affect the physical and chemical properties of elements. It manifests itself on macroscopic scales by. Find out how core electrons repel outer electrons and. Electrons in an s s orbital can shield p p. Electron Shielding Effect.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog Electron Shielding Effect Find out how core electrons repel outer electrons and. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Learn how it affects ionization. learn. Electron Shielding Effect.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Electron Shielding Effect Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. It manifests itself on macroscopic scales by. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an s s orbital can shield p p electrons at the same energy level. Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 Electron Shielding Effect Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Learn how it affects ionization. electron shielding is the phenomenon where inner electrons reduce. Electron Shielding Effect.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 Electron Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Learn how it affects ionization. learn how electron shielding affects the potential energy and orbital energies of atoms and ions. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m. Electron Shielding Effect.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free Electron Shielding Effect learn how electron shielding and penetration affect the physical and chemical properties of elements. Learn how it affects ionization. It manifests itself on macroscopic scales by. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. learn how electron shielding affects the potential energy and orbital energies of atoms and. Electron Shielding Effect.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free Electron Shielding Effect electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. It manifests itself on macroscopic scales by. Learn how it affects ionization. Effective nuclear charge, zeff, experienced by an electron is less than the actual. Electron Shielding Effect.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Electron Shielding Effect Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. learn how electron shielding and penetration affect the physical and chemical properties of elements. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. It manifests itself on macroscopic scales by. . Electron Shielding Effect.
From www.youtube.com
Chapter 3 Shielding Effect and Atomic Size YouTube Electron Shielding Effect learn how electron shielding and penetration affect the physical and chemical properties of elements. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). Learn how it affects ionization. learn how electron shielding affects the potential energy and orbital energies of atoms and. Electron Shielding Effect.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free Electron Shielding Effect Find out how core electrons repel outer electrons and. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). Learn how it affects ionization. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape. Electron Shielding Effect.
From chemisfast.blogspot.com
shielding effect Slater's rules and its limitations. PG.CHEMEASY Electron Shielding Effect Find out how core electrons repel outer electrons and. electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. learn how electron shielding and penetration affect the physical and chemical properties of elements. Effective. Electron Shielding Effect.
From mungfali.com
What Is Shielding Effect Electron Shielding Effect Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). learn how electron shielding affects the potential energy and orbital energies of atoms and ions. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples.. Electron Shielding Effect.
From mungfali.com
What Is Shielding Effect Electron Shielding Effect Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. learn how electron shielding and penetration affect the physical and chemical properties of elements. electron shielding is the phenomenon where inner. Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3652853 Electron Shielding Effect electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. learn how electron shielding and penetration affect the physical and chemical properties of elements. Learn how it affects ionization. It manifests itself on macroscopic scales by. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations. Electron Shielding Effect.
From www.toppr.com
How are shielding effect and atomic radius related? Electron Shielding Effect electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Find out how core electrons repel outer electrons and. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Electrons in an s s orbital can shield p p electrons at the same energy level. Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download Electron Shielding Effect Find out how core electrons repel outer electrons and. electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Learn how it affects ionization. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). Electrons in an s s. Electron Shielding Effect.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint Electron Shielding Effect Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. learn how electron shielding and penetration affect the. Electron Shielding Effect.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Electron Shielding Effect electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Learn how it affects ionization. learn how electron shielding affects the potential energy and orbital energies of atoms and ions. Find out how core electrons repel outer electrons and. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant. Electron Shielding Effect.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Electron Shielding Effect learn how electron shielding and penetration affect the physical and chemical properties of elements. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Electrons. Electron Shielding Effect.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 Electron Shielding Effect Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. learn how electron shielding and penetration affect the physical and chemical properties of elements. electron shielding is the phenomenon where inner electrons reduce the attractive force on outer electrons. Electrons in an s s orbital can shield p p. Electron Shielding Effect.
From www.slideshare.net
Chapter 6 Periodic Table Electron Shielding Effect learn how electron shielding and penetration affect the physical and chemical properties of elements. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n. Electron Shielding Effect.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity Electron Shielding Effect It manifests itself on macroscopic scales by. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. learn how electron shielding affects the potential energy and orbital energies of atoms and ions.. Electron Shielding Effect.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube Electron Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. It manifests itself on macroscopic scales by. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). Find out how core electrons repel outer electrons and. . Electron Shielding Effect.
From chemisfast.blogspot.com
shielding effect Slater's rules and its limitations. PG.CHEMEASY Electron Shielding Effect Find out how core electrons repel outer electrons and. It manifests itself on macroscopic scales by. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital.. Electron Shielding Effect.
From slidetodoc.com
Chapter 6 THE PERIODIC TABLE 1 Introduction Activity Electron Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. It manifests itself on macroscopic scales by. learn how electron shielding affects the potential energy and orbital energies of atoms and ions. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n. Electron Shielding Effect.
From thechemistrynotes.com
Shielding effect Electron Shielding Effect Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). Find out how core electrons repel outer electrons and.. Electron Shielding Effect.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electron Shielding Effect Learn how it affects ionization. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in different orbitals have different wavefunctions and therefore different radial distributions and probabilities (defined by quantum numbers n and m l around the nucleus). electron shielding is the phenomenon where inner electrons reduce the attractive. Electron Shielding Effect.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free Electron Shielding Effect learn how electron shielding and penetration affect the physical and chemical properties of elements. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Electrons in different orbitals have different wavefunctions and. Electron Shielding Effect.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free Electron Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. learn how electron shielding and penetration affect the physical and chemical properties of elements. Find out how to calculate the effective nuclear charge (zeff) and the shielding constant (s) using equations and examples. Electrons in an s s orbital can shield. Electron Shielding Effect.