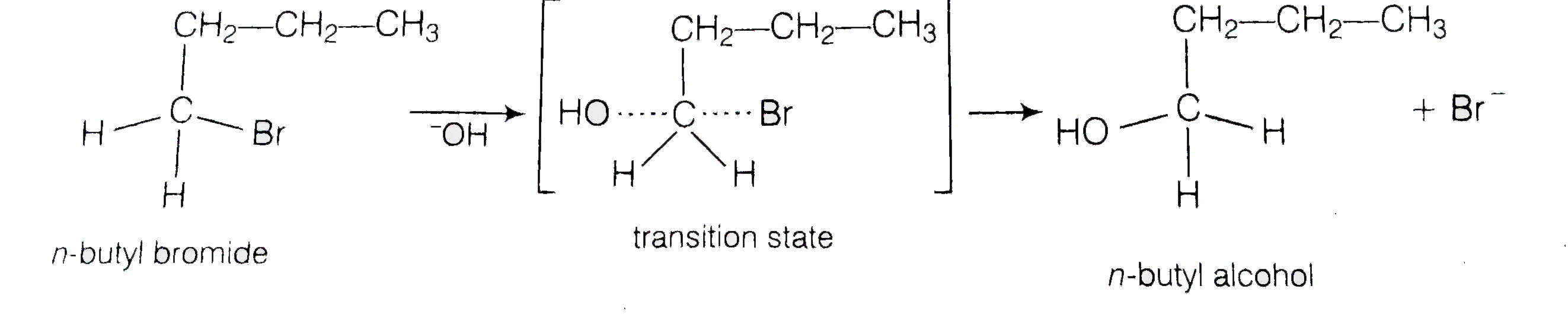Ethyl Bromide With Hydroxide . show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. How are these two reactions. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and.
from www.doubtnut.com
the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. How are these two reactions. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and.
tertButylbromide reacts with aq. NaOH by S(N)1 mechanism while nbuty
Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. How are these two reactions.
From brainly.in
Name the organic compound formed when ethyl bromide reacts with Ethyl Bromide With Hydroxide the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. show the structures of the alkenes that. Ethyl Bromide With Hydroxide.
From www.youtube.com
tertButybromide to isobutylbromide Organic chemistry conversions for Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. How are these two reactions. the zaitsev rule is a good predictor. Ethyl Bromide With Hydroxide.
From www.pdfprof.com
how to make ethyl ethanoate from ethanol Ethyl Bromide With Hydroxide ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work,. Ethyl Bromide With Hydroxide.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. this page looks. Ethyl Bromide With Hydroxide.
From www.fishersci.fi
2Phenoxyethyl bromide, 98, Thermo Scientific Chemicals Fisher Ethyl Bromide With Hydroxide this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. show the structures of the alkenes that could be. Ethyl Bromide With Hydroxide.
From www.masterorganicchemistry.com
The SN2 Reaction Mechanism Master Organic Chemistry Ethyl Bromide With Hydroxide How are these two reactions. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work,. Ethyl Bromide With Hydroxide.
From www.meritnation.com
since when ethyl Bromide is treated with alcoholic potassium hydroxide Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. How are these two reactions. the zaitsev rule is a good predictor. Ethyl Bromide With Hydroxide.
From www.numerade.com
SOLVED The reaction between ethyl bromide (C2H5Br) and hydroxide ion Ethyl Bromide With Hydroxide ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. How are these two reactions.. Ethyl Bromide With Hydroxide.
From www.scribd.com
Mechanism and Products of the Elimination Reaction of Ethyl Bromide Ethyl Bromide With Hydroxide this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. . Ethyl Bromide With Hydroxide.
From www.toppr.com
What happens when(i) isopropyl bromide is hea Ethyl Bromide With Hydroxide ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work,. Ethyl Bromide With Hydroxide.
From www.doubtnut.com
tertButylbromide reacts with aq. NaOH by S(N)1 mechanism while nbuty Ethyl Bromide With Hydroxide ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. this page looks. Ethyl Bromide With Hydroxide.
From brainly.com
Consider the reaction of an alkyl bromide and hydroxide ion. OH Draw Ethyl Bromide With Hydroxide for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as. Ethyl Bromide With Hydroxide.
From www.youtube.com
nButyl bromide Synthesis by SN2 Reaction YouTube Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. the zaitsev rule is a good predictor for simple elimination reactions of. Ethyl Bromide With Hydroxide.
From www.alamy.com
Gel electrophoresis ethidium bromide hires stock photography and Ethyl Bromide With Hydroxide show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. How are these two reactions. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. this page. Ethyl Bromide With Hydroxide.
From www.knowledgeboat.com
Chapter 8 Organic Chemistry Solutions for Class 10 Viraf J Dalal Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. show the structures. Ethyl Bromide With Hydroxide.
From askfilo.com
Ethyl bromide on boiling with alcoholic solutions of sodium hydroxide for.. Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and. Ethyl Bromide With Hydroxide.
From www.masterorganicchemistry.com
Alkyl Halide Reaction Map 14 Key Reactions Of Alkyl Halides Ethyl Bromide With Hydroxide ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. How are these two reactions. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong.. Ethyl Bromide With Hydroxide.
From www.chegg.com
Solved 5. The reaction of ethyl bromide with sodium Ethyl Bromide With Hydroxide How are these two reactions. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. show the structures of the alkenes that could be formed from each by. Ethyl Bromide With Hydroxide.
From www.chegg.com
Solved Consider the reaction of an alkyl bromide and Ethyl Bromide With Hydroxide show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. How are these two reactions. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene.. Ethyl Bromide With Hydroxide.
From www.fishersci.se
Ethyltriphenylphosphonium bromide, 98, Thermo Scientific Chemicals Ethyl Bromide With Hydroxide the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. How are these two reactions. this page gives you the facts and simple, uncluttered mechanisms for the. Ethyl Bromide With Hydroxide.
From www.toppr.com
Q. 19. What is the action of the following on ethyl bromide (i Ethyl Bromide With Hydroxide How are these two reactions. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium.. Ethyl Bromide With Hydroxide.
From www.chemtube3d.com
BaseCatalysed Bromination of Ketones Summary Ethyl Bromide With Hydroxide How are these two reactions. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. for example, the classic. Ethyl Bromide With Hydroxide.
From www.embibe.com
Identify the starting amide which givespmethyl aniline on reaction with Ethyl Bromide With Hydroxide this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small. Ethyl Bromide With Hydroxide.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene.. Ethyl Bromide With Hydroxide.
From brainly.in
how will you convert ethane into ethyl bromide Brainly.in Ethyl Bromide With Hydroxide the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. ethyl bromide can react with sodium hydroxide to give ethanol or. Ethyl Bromide With Hydroxide.
From www.researchgate.net
(PDF) Dimeric ethyltin(IV)dibromidehydroxide N , N dimethylformamide Ethyl Bromide With Hydroxide ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. How are these two reactions. this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes. Ethyl Bromide With Hydroxide.
From exoytvgui.blob.core.windows.net
Dr Sebi Bromide Benefits at Amanda Lee blog Ethyl Bromide With Hydroxide the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. show the structures of the alkenes that. Ethyl Bromide With Hydroxide.
From www.indiamart.com
Ethyl Bromide, For Tamsulosin, 1 Kg, Rs 5500 /kilogram Visa Chem Ethyl Bromide With Hydroxide How are these two reactions. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. the zaitsev rule is a good predictor for simple elimination reactions of alkyl. Ethyl Bromide With Hydroxide.
From chem.libretexts.org
Hydroxyl Group Substitution Chemistry LibreTexts Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. How are these two reactions. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl. Ethyl Bromide With Hydroxide.
From solvedlib.com
Consider the reaction of an alkyl bromide with hydrox… SolvedLib Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with.. Ethyl Bromide With Hydroxide.
From www.numerade.com
SOLVED The mechanism for the E2 reaction of ethyl bromide with Ethyl Bromide With Hydroxide show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. How are these two reactions. this page. Ethyl Bromide With Hydroxide.
From www.inforad.co.kr
화합물 사전 브로모에테인(Bromoethane Ethylbromide) Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. show the structures. Ethyl Bromide With Hydroxide.
From www.doubtnut.com
[Odia] How does ethyl bromide react with sodium ethoxide? Ethyl Bromide With Hydroxide for example, the classic way to make diethyl ether is to treat the ethyl halide (the chloride, bromide, or iodide all work, but not the fluoride) with. How are these two reactions. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. this page. Ethyl Bromide With Hydroxide.
From chem.libretexts.org
24.S Amines and Heterocycles (Summary) Chemistry LibreTexts Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. the zaitsev rule is a good predictor for simple elimination reactions of alkyl chlorides, bromides and iodides as long as relatively small strong. ethyl bromide can react with sodium hydroxide to give ethanol or ethylene. How are these two. Ethyl Bromide With Hydroxide.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Ethyl Bromide With Hydroxide this page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and. show the structures of the alkenes that could be formed from each by antarafacial \(e2\) elimination of one mole of hydrogen bromide with. this page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from. Ethyl Bromide With Hydroxide.