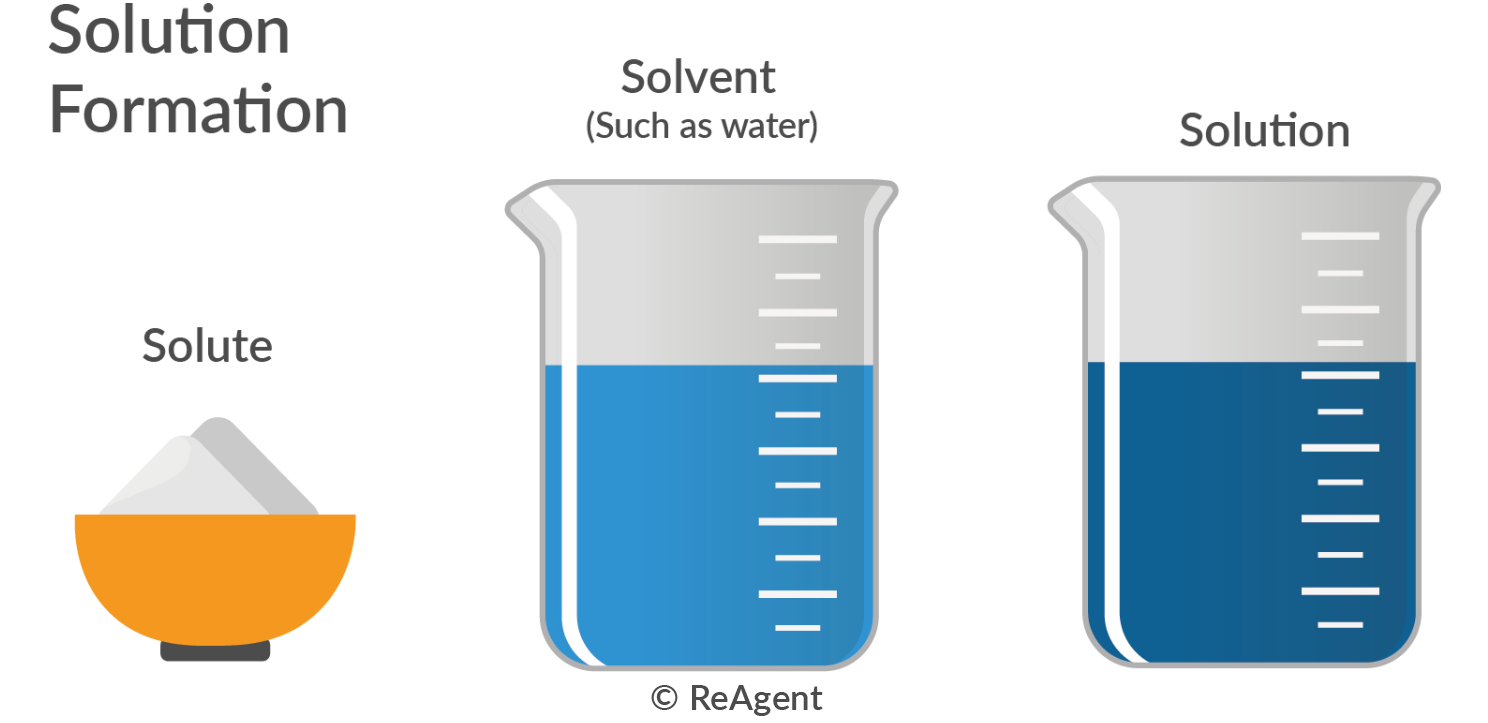
Example Of A Solution In Chemistry . A solution consists of a solute and a solvent. The amount of solute that can be dissolved in a solvent is called. The solvent is present in greater quantities than the solute in fluid. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. Other examples of solutions include: A solution is a homogeneous mixture of two or more pure substances. The solute is the substance that is dissolved in the solvent. A solute is defined as a substance that dissolves in a solution. The concentration of a chemical solution is a. The substance that is in a large amount in the solution is called the.
from www.chemicals.co.uk
The solute is the substance that is dissolved in the solvent. The amount of solute that can be dissolved in a solvent is called. Other examples of solutions include: A solution consists of a solute and a solvent. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. A solution is a homogeneous mixture of two or more pure substances. The substance that is in a large amount in the solution is called the. The concentration of a chemical solution is a. A solute is defined as a substance that dissolves in a solution. The solvent is present in greater quantities than the solute in fluid.
What Is A Standard Solution? The Chemistry Blog
Example Of A Solution In Chemistry The amount of solute that can be dissolved in a solvent is called. A solution is a homogeneous mixture of two or more pure substances. Other examples of solutions include: The amount of solute that can be dissolved in a solvent is called. The concentration of a chemical solution is a. A solute is defined as a substance that dissolves in a solution. The solute is the substance that is dissolved in the solvent. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. A solution consists of a solute and a solvent. The substance that is in a large amount in the solution is called the. The solvent is present in greater quantities than the solute in fluid.
From sites.google.com
9/8/15 Miss Day's Science Class Blog Example Of A Solution In Chemistry Other examples of solutions include: A solute is defined as a substance that dissolves in a solution. A solution consists of a solute and a solvent. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The amount of solute that can be dissolved in a solvent is called.. Example Of A Solution In Chemistry.
From study.com
Saturated Solution Definition & Examples Lesson Example Of A Solution In Chemistry A solution is a homogeneous mixture of two or more pure substances. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The amount of solute that can be dissolved in a solvent is called. Other examples of solutions include: The solvent is present in greater quantities than the. Example Of A Solution In Chemistry.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples Example Of A Solution In Chemistry The amount of solute that can be dissolved in a solvent is called. A solution is a homogeneous mixture of two or more pure substances. A solute is defined as a substance that dissolves in a solution. A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. The solvent is. Example Of A Solution In Chemistry.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Example Of A Solution In Chemistry The substance that is in a large amount in the solution is called the. The amount of solute that can be dissolved in a solvent is called. The concentration of a chemical solution is a. A solution consists of a solute and a solvent. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can. Example Of A Solution In Chemistry.
From www.teachoo.com
What is a Mixture? Types of Mixtures Chemistry Teachoo Example Of A Solution In Chemistry The concentration of a chemical solution is a. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The solute is the substance that is dissolved in the solvent. A solute is defined as a substance that dissolves in a solution. The substance that is in a large amount. Example Of A Solution In Chemistry.
From library.fiveable.me
Solutions and Mixtures AP Chemistry Class Notes Fiveable Example Of A Solution In Chemistry The amount of solute that can be dissolved in a solvent is called. Other examples of solutions include: The concentration of a chemical solution is a. The substance that is in a large amount in the solution is called the. A solution consists of a solute and a solvent. A solute is defined as a substance that dissolves in a. Example Of A Solution In Chemistry.
From www.slideserve.com
PPT Intro to Chemistry PowerPoint Presentation, free download ID Example Of A Solution In Chemistry The amount of solute that can be dissolved in a solvent is called. Other examples of solutions include: A solution consists of a solute and a solvent. The solvent is present in greater quantities than the solute in fluid. The solute is the substance that is dissolved in the solvent. Solution, in chemistry, a homogenous mixture of two or more. Example Of A Solution In Chemistry.
From www.geeksforgeeks.org
Solution Definition, Types, Properties, Examples, and FAQs Example Of A Solution In Chemistry Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. A solute is defined as a substance that dissolves in a solution. The substance that is in a large amount in the solution is called the. A solution consists of a solute and a solvent. A solution is a. Example Of A Solution In Chemistry.
From www.slideserve.com
PPT Types of Solutions PowerPoint Presentation, free download ID Example Of A Solution In Chemistry A solute is defined as a substance that dissolves in a solution. Other examples of solutions include: The solute is the substance that is dissolved in the solvent. The amount of solute that can be dissolved in a solvent is called. A solution is a homogeneous mixture of two or more pure substances. The concentration of a chemical solution is. Example Of A Solution In Chemistry.
From www.vrogue.co
10 Examples Of Solutions vrogue.co Example Of A Solution In Chemistry A solution is a homogeneous mixture of two or more pure substances. The substance that is in a large amount in the solution is called the. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The solute is the substance that is dissolved in the solvent. A solution. Example Of A Solution In Chemistry.
From www.thoughtco.com
Solution Chemistry Glossary Definition Example Of A Solution In Chemistry The substance that is in a large amount in the solution is called the. Other examples of solutions include: The solute is the substance that is dissolved in the solvent. The solvent is present in greater quantities than the solute in fluid. A solution consists of a solute and a solvent. A solution is a homogeneous mixture of two or. Example Of A Solution In Chemistry.
From www.thoughtco.com
Aqueous Solution Definition in Chemistry Example Of A Solution In Chemistry A solution is a homogeneous mixture of two or more pure substances. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. A solute is defined as a substance that dissolves in a solution. Other examples of solutions include: The solute is the substance that is dissolved in the. Example Of A Solution In Chemistry.
From brunofuga.adv.br
Solution Chemistry Definition, Types, Examples, 52 OFF Example Of A Solution In Chemistry The concentration of a chemical solution is a. The amount of solute that can be dissolved in a solvent is called. A solution consists of a solute and a solvent. Other examples of solutions include: Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. A solute is defined. Example Of A Solution In Chemistry.
From sciencenotes.org
Solution Definition in Chemistry Example Of A Solution In Chemistry Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The amount of solute that can be dissolved in a solvent is called. Other examples of solutions include: A solution consists of a solute and a solvent. A solution is a homogeneous mixture of two or more pure substances.. Example Of A Solution In Chemistry.
From sciencenotes.org
Supersaturated Solution Definition and Examples Example Of A Solution In Chemistry Other examples of solutions include: A solute is defined as a substance that dissolves in a solution. The amount of solute that can be dissolved in a solvent is called. The solute is the substance that is dissolved in the solvent. A solution consists of a solute and a solvent. The solvent is present in greater quantities than the solute. Example Of A Solution In Chemistry.
From study.com
What is a Solution in Science? Definition & Examples Video & Lesson Example Of A Solution In Chemistry Other examples of solutions include: Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The substance that is in a large amount in the solution is called the. The amount of solute that can be dissolved in a solvent is called. A solution consists of a solute and. Example Of A Solution In Chemistry.
From proper-cooking.info
Solution Chemistry Examples Example Of A Solution In Chemistry A solute is defined as a substance that dissolves in a solution. The substance that is in a large amount in the solution is called the. The amount of solute that can be dissolved in a solvent is called. The solute is the substance that is dissolved in the solvent. A solution is a homogeneous mixture of two or more. Example Of A Solution In Chemistry.
From gbu-presnenskij.ru
Solution Chemistry Definition, Types, Examples, 47 OFF Example Of A Solution In Chemistry The amount of solute that can be dissolved in a solvent is called. The concentration of a chemical solution is a. The solute is the substance that is dissolved in the solvent. A solution is a homogeneous mixture of two or more pure substances. Other examples of solutions include: The solvent is present in greater quantities than the solute in. Example Of A Solution In Chemistry.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Example Of A Solution In Chemistry A solution is a homogeneous mixture of two or more pure substances. Other examples of solutions include: A solute is defined as a substance that dissolves in a solution. The amount of solute that can be dissolved in a solvent is called. A solution consists of a solute and a solvent. The substance that is in a large amount in. Example Of A Solution In Chemistry.
From sciencenotes.org
What Is a Solvent? Definition and Examples Example Of A Solution In Chemistry A solute is defined as a substance that dissolves in a solution. The solvent is present in greater quantities than the solute in fluid. The substance that is in a large amount in the solution is called the. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The. Example Of A Solution In Chemistry.
From examples.yourdictionary.com
Examples of Heterogeneous Mixtures Types Made Simple YourDictionary Example Of A Solution In Chemistry The concentration of a chemical solution is a. The solute is the substance that is dissolved in the solvent. The amount of solute that can be dissolved in a solvent is called. A solution is a homogeneous mixture of two or more pure substances. A solute is defined as a substance that dissolves in a solution. The solvent is present. Example Of A Solution In Chemistry.
From www.studypug.com
Introduction to Solution Chemistry Explore Solubility StudyPug Example Of A Solution In Chemistry A solution is a homogeneous mixture of two or more pure substances. A solution consists of a solute and a solvent. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The concentration of a chemical solution is a. A solute is defined as a substance that dissolves in. Example Of A Solution In Chemistry.
From www.chemicals.co.uk
What Is A Standard Solution? The Chemistry Blog Example Of A Solution In Chemistry The solute is the substance that is dissolved in the solvent. A solute is defined as a substance that dissolves in a solution. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The solvent is present in greater quantities than the solute in fluid. A solution consists of. Example Of A Solution In Chemistry.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Example Of A Solution In Chemistry Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. Other examples of solutions include: A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. The substance that is in a large amount in the solution is called the.. Example Of A Solution In Chemistry.
From examples.yourdictionary.com
Examples of Saturated Solution YourDictionary Example Of A Solution In Chemistry Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The solute is the substance that is dissolved in the solvent. The amount of solute that can be dissolved in a solvent is called. The concentration of a chemical solution is a. A solution consists of a solute and. Example Of A Solution In Chemistry.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID Example Of A Solution In Chemistry A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. Other examples of solutions include: The substance that is in a large amount in the solution is called the. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to.. Example Of A Solution In Chemistry.
From chemistrytalk.org
What is a Solution in Chemistry? ChemTalk Example Of A Solution In Chemistry Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. The solvent is present in greater quantities than the solute in fluid. The solute is the substance that is dissolved in the solvent. The substance that is in a large amount in the solution is called the. A solute. Example Of A Solution In Chemistry.
From www.pinterest.com
Aqueous Solution Solutions, Chemistry experiments, Chemistry study guide Example Of A Solution In Chemistry The substance that is in a large amount in the solution is called the. Other examples of solutions include: The solute is the substance that is dissolved in the solvent. A solute is defined as a substance that dissolves in a solution. The concentration of a chemical solution is a. Solution, in chemistry, a homogenous mixture of two or more. Example Of A Solution In Chemistry.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 1 activity for kids Example Of A Solution In Chemistry The substance that is in a large amount in the solution is called the. The concentration of a chemical solution is a. A solution is a homogeneous mixture of two or more pure substances. A solute is defined as a substance that dissolves in a solution. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts. Example Of A Solution In Chemistry.
From www.bharatagritech.com
Solution Chemistry Definition, Types, Examples, 53 OFF Example Of A Solution In Chemistry Other examples of solutions include: The solvent is present in greater quantities than the solute in fluid. The substance that is in a large amount in the solution is called the. The concentration of a chemical solution is a. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to.. Example Of A Solution In Chemistry.
From gbu-presnenskij.ru
Solution Chemistry Definition, Types, Examples, 57 OFF Example Of A Solution In Chemistry The solvent is present in greater quantities than the solute in fluid. A solute is defined as a substance that dissolves in a solution. Other examples of solutions include: A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. The concentration of a chemical solution is a. The amount of. Example Of A Solution In Chemistry.
From sciencenotes.org
What Is a Homogeneous Mixture? Definition and Examples Example Of A Solution In Chemistry Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to. A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. A solution is a homogeneous mixture of two or more pure substances. The solvent is present in greater quantities. Example Of A Solution In Chemistry.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID Example Of A Solution In Chemistry The solvent is present in greater quantities than the solute in fluid. A solution is a homogeneous mixture of two or more pure substances. The concentration of a chemical solution is a. Other examples of solutions include: A solution consists of a solute and a solvent. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts. Example Of A Solution In Chemistry.
From www.youtube.com
9 Types of Solution Chemistry YouTube Example Of A Solution In Chemistry The concentration of a chemical solution is a. A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. The solvent is present in greater quantities than the solute in fluid. A solute is defined as a substance that dissolves in a solution. The amount of solute that can be dissolved. Example Of A Solution In Chemistry.
From ar.inspiredpencil.com
Solution Chemistry Examples Example Of A Solution In Chemistry A solute is defined as a substance that dissolves in a solution. Other examples of solutions include: A solution is a homogeneous mixture of two or more pure substances. The concentration of a chemical solution is a. The substance that is in a large amount in the solution is called the. Solution, in chemistry, a homogenous mixture of two or. Example Of A Solution In Chemistry.