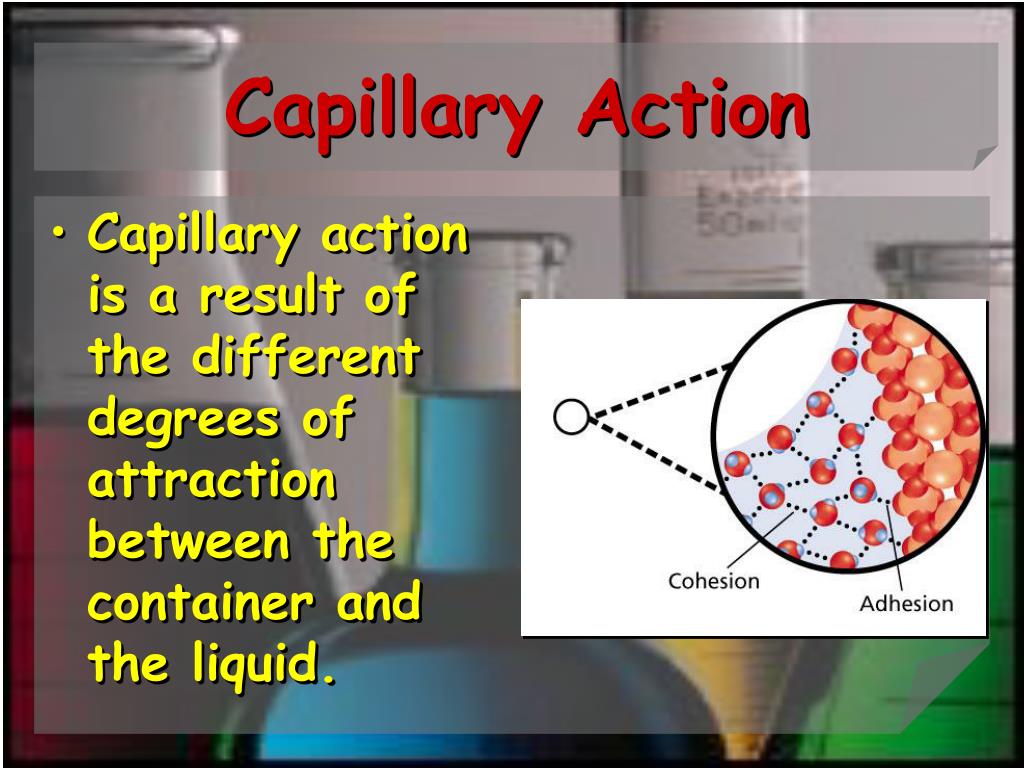
Capillary Chemistry . — capillary action is a phenomenon in which liquid flows through a narrow tube without the assistance of any force. — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. In fact, it often acts in opposition to gravity. Capillary action occurs when the force of adhesion is greater than the force of cohesion. Capillary action is sometimes called capillary motion, capillarity, or wicking. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. This movement does not require the force of gravity to occur. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity.
from www.slideserve.com
— capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. Capillary action occurs when the force of adhesion is greater than the force of cohesion. This movement does not require the force of gravity to occur. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. In fact, it often acts in opposition to gravity. Capillary action is sometimes called capillary motion, capillarity, or wicking. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as.
PPT Chapter 1213 PowerPoint Presentation, free download ID2214763
Capillary Chemistry It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. Capillary action occurs when the force of adhesion is greater than the force of cohesion. — capillary action is a phenomenon in which liquid flows through a narrow tube without the assistance of any force. — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. In fact, it often acts in opposition to gravity. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. Capillary action is sometimes called capillary motion, capillarity, or wicking. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. This movement does not require the force of gravity to occur.
From study.com
Capillary Fluid Exchange Overview & Hydrostatic Pressure Video Capillary Chemistry — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. — capillary action is a phenomenon in which liquid flows through a narrow tube without the. Capillary Chemistry.
From www.showme.com
Diagram of Capillary Bed Science Biology Science, Biology, Organic Capillary Chemistry Capillary action occurs when the force of adhesion is greater than the force of cohesion. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. In fact, it often acts in opposition to gravity. — capillary action is a phenomenon in which liquid flows through a narrow tube. Capillary Chemistry.
From www.youtube.com
How to Calculate the Pressure in Capillary Tubes + Application of Capillary Chemistry Capillary action occurs when the force of adhesion is greater than the force of cohesion. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. Capillary action is sometimes. Capillary Chemistry.
From www.teachersource.com
Capillary Tube Set, Chemistry Educational Innovations, Inc. Capillary Chemistry Capillary action occurs when the force of adhesion is greater than the force of cohesion. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. This movement does not require the force of gravity to occur. — capillary action can be defined. Capillary Chemistry.
From universe84a.com
Capillary Electrophoresis Introduction, instrumentation, procedure Capillary Chemistry — capillary action is a phenomenon in which liquid flows through a narrow tube without the assistance of any force. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. Capillary action occurs when the force of adhesion is greater than the force of cohesion. Capillary action is sometimes called. Capillary Chemistry.
From eduinput.com
What are Capillaries?Types, Mechanism of Action, and Functions Capillary Chemistry — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. This movement does not require the force of gravity to occur. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary.. Capillary Chemistry.
From dxorypcco.blob.core.windows.net
Capillary Tubes Of Blood at Christopher Hollingsworth blog Capillary Chemistry capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. — capillary action is a phenomenon in which liquid flows through a narrow tube without the assistance of any force. Capillary. Capillary Chemistry.
From www.differencebetween.com
Difference Between Surface Tension and Capillary Action Compare the Capillary Chemistry Capillary action is sometimes called capillary motion, capillarity, or wicking. — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. . Capillary Chemistry.
From chem.libretexts.org
Lab 6 Capillary Electrophoresis Chemistry LibreTexts Capillary Chemistry capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a. Capillary Chemistry.
From eduinput.com
7 Examples of Capillarity Capillary Chemistry — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. Capillary action occurs when the force of adhesion is greater than the force of cohesion. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. capillary action is defined. Capillary Chemistry.
From www.britannica.com
Capillary Blood Vessels, Exchange & Function Britannica Capillary Chemistry — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. This movement does not require the force of gravity to occur. Capillary action is sometimes called capillary. Capillary Chemistry.
From lewis-has-ritter.blogspot.com
Capillary Action Is a Term Used to Describe How LewishasRitter Capillary Chemistry — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. This movement does not require the force of gravity to occur. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. Capillary action is sometimes called capillary motion, capillarity, or wicking. . Capillary Chemistry.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Capillary Chemistry It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. capillary action is defined as the spontaneous flow of a liquid into a narrow tube. Capillary Chemistry.
From www.researchgate.net
3 Schematic representation of capillary electrophoresis (CE). The four Capillary Chemistry In fact, it often acts in opposition to gravity. Capillary action is sometimes called capillary motion, capillarity, or wicking. This movement does not require the force of gravity to occur. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. capillary action is defined as the spontaneous. Capillary Chemistry.
From guides.hostos.cuny.edu
Chapter 3 Solids and Liquids CHE 110 Introduction to Chemistry Capillary Chemistry capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. Capillary action is sometimes called capillary motion, capillarity, or wicking. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. It results when cohesive forces, the intermolecular forces in the. Capillary Chemistry.
From www.dreamstime.com
Capillary Columns for Gas Chromatographic Analysis. Equipment for Capillary Chemistry — capillary action is a phenomenon in which liquid flows through a narrow tube without the assistance of any force. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. In fact, it often acts in opposition to gravity. It refers to the ascension of liquid through a capillary tube. Capillary Chemistry.
From www.youtube.com
Definition of a Spotting Capillary in Chemistry Chemistry Concepts Capillary Chemistry — capillary action is a phenomenon in which liquid flows through a narrow tube without the assistance of any force. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. capillary action is defined as the spontaneous flow of a liquid. Capillary Chemistry.
From www.thoughtco.com
An Illustrated Guide to Capillary Fluid Exchange Capillary Chemistry This movement does not require the force of gravity to occur. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. Capillary action is sometimes called capillary motion, capillarity, or wicking. Capillary action occurs when the force of adhesion is greater than the force of cohesion. It results. Capillary Chemistry.
From www.slideserve.com
PPT A.P. Chemistry PowerPoint Presentation, free download ID5696562 Capillary Chemistry capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. This movement does not require the force of gravity to occur. Capillary action occurs when the force of adhesion is greater than the force of cohesion. It refers to the ascension of liquid through a capillary tube due to forces of. Capillary Chemistry.
From thebiologynotes.com
Capillaries Structure, 3 Types, Functions, Diseases Capillary Chemistry In fact, it often acts in opposition to gravity. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. Capillary action occurs when the force of adhesion is greater than the force of cohesion. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and. Capillary Chemistry.
From sketchfab.com
Capillary Chemistry 3D model by Mieke Roth (miekeroth) [71c4c27 Capillary Chemistry It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. Capillary action is sometimes called capillary motion, capillarity, or wicking. This. Capillary Chemistry.
From www.dreamstime.com
Capillary Columns for Gas Chromatographic Analysis. Equipment for Capillary Chemistry Capillary action occurs when the force of adhesion is greater than the force of cohesion. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. Capillary action is sometimes called capillary motion,. Capillary Chemistry.
From www.thoughtco.com
An Illustrated Guide to Capillary Fluid Exchange Capillary Chemistry It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. — capillary action is the phenomenon in which liquids rise up. Capillary Chemistry.
From www.youtube.com
Capillary action is the ability of a liquid to flow in narrow spaces Capillary Chemistry — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. Capillary action occurs when the force of adhesion is greater than the force of cohesion. In fact, it often acts in opposition to gravity. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and. Capillary Chemistry.
From www.pinterest.com
Simplifying the chemistry that makes cardiovascular capillaries work Capillary Chemistry This movement does not require the force of gravity to occur. Capillary action occurs when the force of adhesion is greater than the force of cohesion. Capillary action is sometimes called capillary motion, capillarity, or wicking. In fact, it often acts in opposition to gravity. — capillary action (or capillarity) describes the ability of a liquid to flow against. Capillary Chemistry.
From www.researchgate.net
(a) Sketch of capillary rise in a vertical tube. (b) Sketch of the Capillary Chemistry — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. — capillary action is a phenomenon in which liquid flows through a narrow tube without the assistance of. Capillary Chemistry.
From www.thoughtco.com
What Is Capillary Action? Definition and Examples Capillary Chemistry This movement does not require the force of gravity to occur. — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. Capillary action occurs when the force of adhesion is greater than the force of cohesion. — capillary action can be defined as the ascension of liquids. Capillary Chemistry.
From study.com
Capillary Electrophoresis Characteristics & Examples Lesson Capillary Chemistry — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between a liquid and the surface of the capillary. capillary action is defined as the spontaneous flow of a liquid into a. Capillary Chemistry.
From sciencenotes.org
Capillary Action What It Is and How It Works Capillary Chemistry — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. It results when cohesive forces, the intermolecular forces in the liquid, are weaker than adhesive forces, the attraction between. Capillary Chemistry.
From medmovie.com
Capillaries Capillary Chemistry Capillary action occurs when the force of adhesion is greater than the force of cohesion. In fact, it often acts in opposition to gravity. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or. Capillary Chemistry.
From labsuppliesusa.com
Glass MicroHematocrit Capillary Tubes (1000/bx) KLM Bio Scientific Capillary Chemistry Capillary action is sometimes called capillary motion, capillarity, or wicking. — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. In fact, it often acts in opposition to gravity. — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance. Capillary Chemistry.
From www.pnas.org
Bioinspired inner microstructured tube controlled capillary rise PNAS Capillary Chemistry capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. Capillary action is sometimes called capillary motion, capillarity, or wicking. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. — capillary action is the phenomenon in which. Capillary Chemistry.
From chem.libretexts.org
Capillary Action Chemistry LibreTexts Capillary Chemistry — capillary action is a phenomenon in which liquid flows through a narrow tube without the assistance of any force. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. In fact, it often acts in opposition to gravity. — capillary action (or capillarity) describes the ability of a. Capillary Chemistry.
From www.slideserve.com
PPT Chapter 1213 PowerPoint Presentation, free download ID2214763 Capillary Chemistry — capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to. — capillary action is the phenomenon in which liquids rise up into a narrow tube called a capillary. capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. In. Capillary Chemistry.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Capillary Chemistry Capillary action is sometimes called capillary motion, capillarity, or wicking. It refers to the ascension of liquid through a capillary tube due to forces of adhesion and cohesion and, typically, against gravity. — capillary action (or capillarity) describes the ability of a liquid to flow against gravity in a narrow space such as. — capillary action can be. Capillary Chemistry.