What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases . — when the vapour pressure equals atmospheric pressure, the liquid boils. As elevation increases, atmospheric pressure decreases. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point of a liquid depends on. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. Greater than one atmosphere, the boiling point of the liquid is. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which.
from mavink.com
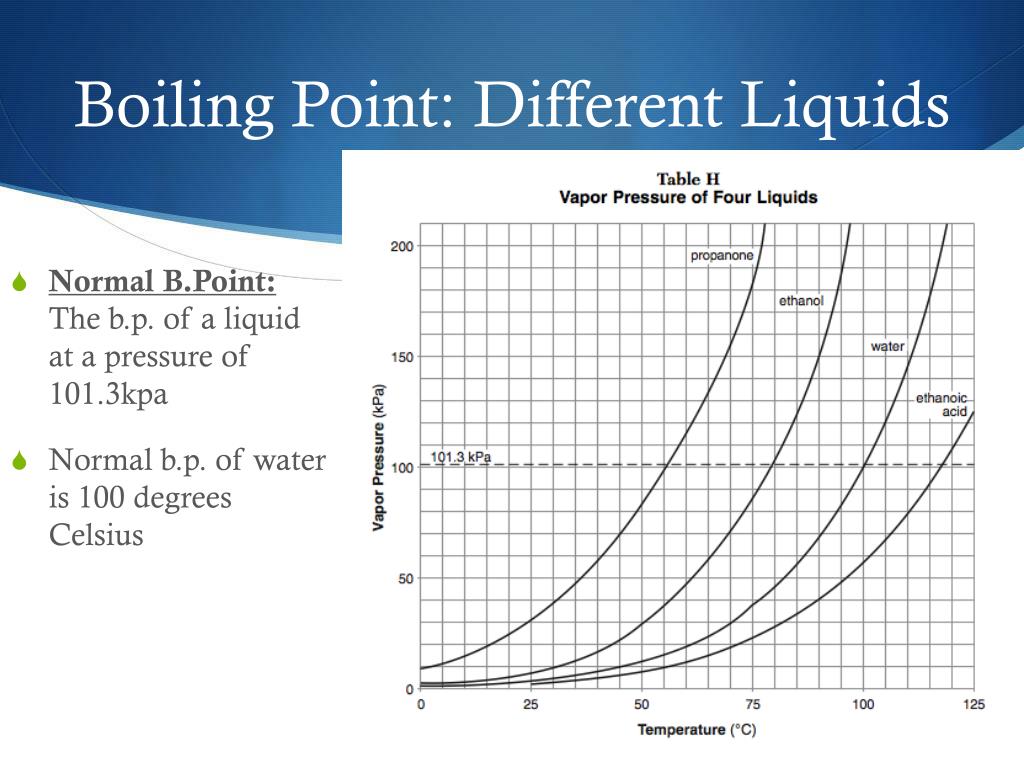
equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. As elevation increases, atmospheric pressure decreases. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. — when the vapour pressure equals atmospheric pressure, the liquid boils. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. The boiling point of a liquid depends on. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. Greater than one atmosphere, the boiling point of the liquid is.
Water Boiling Point Pressure Chart
What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — when the vapour pressure equals atmospheric pressure, the liquid boils. — when the vapour pressure equals atmospheric pressure, the liquid boils. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. The boiling point of a liquid depends on. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Greater than one atmosphere, the boiling point of the liquid is. As elevation increases, atmospheric pressure decreases. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure.
From www.slideshare.net
Air pressure What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases As elevation increases, atmospheric pressure decreases. Greater than one atmosphere, the boiling point of the liquid is. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of a liquid depends on. . What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From slideplayer.com
Boiling points and Intermolecular Forces ppt download What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. equal to one atmosphere, the boiling point of a liquid is called the normal boiling. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. Greater than one atmosphere, the boiling point of the liquid is. — for example, for water,. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From schematiclibzeugma77.z5.web.core.windows.net
Boiling Point And Pressure Chart What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases As elevation increases, atmospheric pressure decreases. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. — the. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From byjus.com
How does external pressure affect the boiling point What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases Greater than one atmosphere, the boiling point of the liquid is. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. As elevation increases, atmospheric pressure decreases. — when the vapour pressure equals atmospheric pressure, the liquid boils. — when the vapor pressure inside the bubbles is equal to the external. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From ar.inspiredpencil.com
Boiling Point Elevation Equation What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — when the vapour pressure equals atmospheric pressure, the liquid boils. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — the boiling point is reached when the vapor pressure of a. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — when the vapour pressure equals atmospheric pressure, the liquid boils. The boiling point of a liquid depends on. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. As elevation increases, atmospheric pressure decreases. Greater than one atmosphere, the boiling point of the liquid is. — the boiling point. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases Greater than one atmosphere, the boiling point of the liquid is. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From exoitchwi.blob.core.windows.net
Boiling Point 120 Degrees at Elaine Briggs blog What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases As elevation increases, atmospheric pressure decreases. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From facts.net
20 Fascinating Facts About Boiling Point What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. — when the vapour pressure equals atmospheric pressure, the liquid boils. As elevation increases, atmospheric pressure. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.chegg.com
Solved 9) Based on the figure below, what is the boiling What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases The boiling point of a liquid depends on. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Greater than one atmosphere, the boiling point of the liquid is. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. the normal boiling point (also. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. As elevation increases, atmospheric pressure decreases. — when the vapour pressure equals atmospheric pressure, the liquid. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From mavink.com
Water Boiling Point Pressure Chart What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure.. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. Greater than one atmosphere, the boiling point of the liquid is. As elevation increases, atmospheric pressure decreases. . What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.alamy.com
Atmospheric pressure alters the boiling point of water Stock Photo Alamy What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. As elevation increases, atmospheric pressure decreases. The boiling point of a liquid depends on. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. equal to. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From in.pinterest.com
How does altitude affect boiling? Vapor pressure and Atmospheric What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases The boiling point of a liquid depends on. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — the boiling point of water is the. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases Greater than one atmosphere, the boiling point of the liquid is. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — for example, for water, the boiling point is 100ºc at a pressure. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From sciencenotes.org
How to Boil Water at Room Temperature What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From schematiclibxylenol123.z22.web.core.windows.net
Diagram Of Atmospheric Pressure What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases Greater than one atmosphere, the boiling point of the liquid is. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. The boiling point of a liquid depends on. — for example, for water, the boiling point is 100ºc at a pressure of 1. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From mavink.com
Water Boiling Point Pressure Chart What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — when the vapour pressure equals atmospheric pressure, the liquid boils. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point of a liquid depends on. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.slideserve.com
PPT To learn about atmospheric pressure and how barometers work To What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. Greater than one atmosphere, the boiling point of the liquid is. — for example, for water, the boiling point is 100ºc at a. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From dxofxnylq.blob.core.windows.net
What Happens To The Boiling Point Of Liquid When Atmospheric Pressure What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases As elevation increases, atmospheric pressure decreases. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The boiling point of a liquid depends on. equal to. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From ponasa.condesan-ecoandes.org
Water Boiling Point Vs Pressure Chart Ponasa What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — for example, for water, the boiling point is 100ºc at a pressure of 1 atm. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point.. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.vrogue.co
How Do Atmospheric Pressure And Elevation Affect Boil vrogue.co What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure,. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From askfilo.com
The Boiling point of water at normal atmospheric pressure is Filo What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. — when the vapour pressure equals atmospheric pressure, the liquid boils. — for example, for. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.acurite.com
Atmospheric Pressure AcuRite What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases Greater than one atmosphere, the boiling point of the liquid is. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — when the vapour pressure equals atmospheric pressure, the liquid boils. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. The boiling point of a liquid depends on. — for example, for water, the boiling point is 100ºc at a pressure. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.worksheetsplanet.com
What is Boiling Point What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. The boiling point of a liquid depends on. the normal boiling point (also called the atmospheric. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From dxofxnylq.blob.core.windows.net
What Happens To The Boiling Point Of Liquid When Atmospheric Pressure What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases Greater than one atmosphere, the boiling point of the liquid is. the normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which. As elevation increases, atmospheric pressure decreases. — for example, for water, the boiling point is 100ºc at a pressure of 1 atm.. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.youtube.com
Boiling point and external pressure YouTube What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases As elevation increases, atmospheric pressure decreases. equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. . What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From exomkbouq.blob.core.windows.net
What Is Boiling Point Elevation Constant at Ronnie Moore blog What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases Greater than one atmosphere, the boiling point of the liquid is. — when the vapour pressure equals atmospheric pressure, the liquid boils. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From mungfali.com
Water Boiling Point Pressure Chart What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Greater than one atmosphere, the boiling point of the liquid is. As elevation increases, atmospheric pressure decreases.. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From www.pinterest.com
Atmospheric pressure and boiling point of water table Boiling point What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid. — the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure.. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.
From exolynvwh.blob.core.windows.net
What Is A Standard Atmospheric Pressure at Micheal Miller blog What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. — the boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. — when the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid.. What Is The Boiling Point Of Water If The External Atmospheric Pressure Decreases.