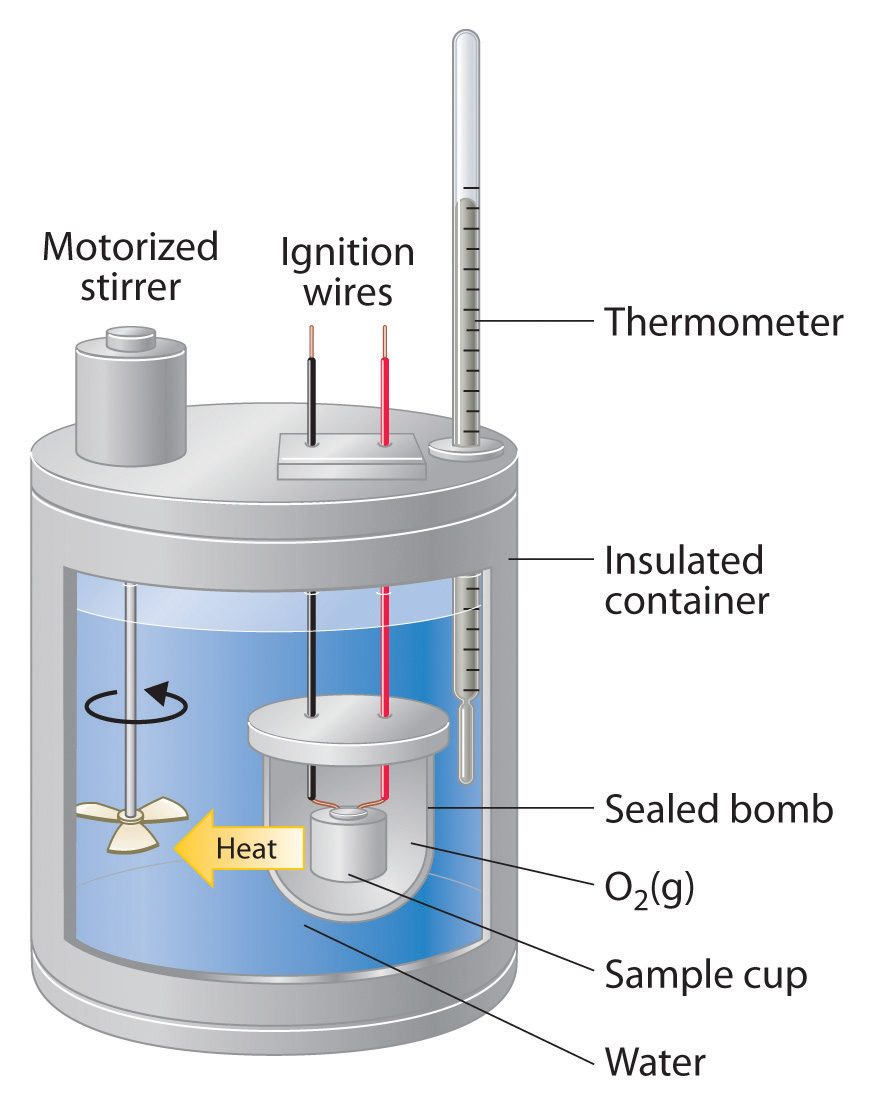
Purpose Of Calorimeter Heat Capacity . The symbol c stands for the specific heat. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and.
from 2012books.lardbucket.org
The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The symbol c stands for the specific heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry is used to measure amounts of heat transferred to. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.
Calorimetry
Purpose Of Calorimeter Heat Capacity Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calorimetry is used to measure amounts of heat transferred to. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. The symbol c stands for the specific heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Purpose Of Calorimeter Heat Capacity To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. In this. Purpose Of Calorimeter Heat Capacity.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Purpose Of Calorimeter Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. The symbol c stands for the specific heat. Q = mcδt, where. Purpose Of Calorimeter Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Purpose Of Calorimeter Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is. Purpose Of Calorimeter Heat Capacity.
From users.highland.edu
Calorimetry Purpose Of Calorimeter Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. The symbol c stands for the specific heat.. Purpose Of Calorimeter Heat Capacity.
From loejdmoqi.blob.core.windows.net
Calorimeter And The Function at Judith Stanton blog Purpose Of Calorimeter Heat Capacity Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. One technique we can use to. Purpose Of Calorimeter Heat Capacity.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Purpose Of Calorimeter Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. To measure the heat capacity of the calorimeter, we first. Purpose Of Calorimeter Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Purpose Of Calorimeter Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.. Purpose Of Calorimeter Heat Capacity.
From joiwmxbys.blob.core.windows.net
Calorimetry With Two Solutions at Angelica Kirkland blog Purpose Of Calorimeter Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. The heat capacity (c) of a body of matter is the quantity. Purpose Of Calorimeter Heat Capacity.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Purpose Of Calorimeter Heat Capacity Calorimetry is used to measure amounts of heat transferred to. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of. Purpose Of Calorimeter Heat Capacity.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Purpose Of Calorimeter Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The heat capacity (c) of a body of matter is the quantity of heat (q) it. Purpose Of Calorimeter Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Purpose Of Calorimeter Heat Capacity Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. In this article, you will learn about calorimetry. Purpose Of Calorimeter Heat Capacity.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Purpose Of Calorimeter Heat Capacity Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry is used to measure amounts of heat transferred to. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. Q =. Purpose Of Calorimeter Heat Capacity.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Purpose Of Calorimeter Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The symbol c stands for the specific heat. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The heat. Purpose Of Calorimeter Heat Capacity.
From www.slideserve.com
PPT CHEM 1011 PowerPoint Presentation, free download ID317228 Purpose Of Calorimeter Heat Capacity Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The symbol c stands for the specific heat. The heat. Purpose Of Calorimeter Heat Capacity.
From www.markedbyteachers.com
The purpose of this experiment was to measure the specific heat Purpose Of Calorimeter Heat Capacity Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. To measure the heat capacity of the calorimeter,. Purpose Of Calorimeter Heat Capacity.
From www.slideserve.com
PPT Purpose of the Experiment PowerPoint Presentation, free download Purpose Of Calorimeter Heat Capacity To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. In this article, you will learn. Purpose Of Calorimeter Heat Capacity.
From www.youtube.com
Thermochemistry Heat Capacity and Calorimetry YouTube Purpose Of Calorimeter Heat Capacity Calorimetry is used to measure amounts of heat transferred to. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The symbol c stands for the specific heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. Purpose Of Calorimeter Heat Capacity.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Purpose Of Calorimeter Heat Capacity Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. Calorimetry is used to measure amounts of heat transferred to. The heat. Purpose Of Calorimeter Heat Capacity.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Purpose Of Calorimeter Heat Capacity To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. One technique we can use to measure the. Purpose Of Calorimeter Heat Capacity.
From www.youtube.com
2.2A Calorimetry, Heat Capacity & Specific Heat Capacity YouTube Purpose Of Calorimeter Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The symbol c stands for the specific heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this article, you will learn. Purpose Of Calorimeter Heat Capacity.
From studylib.net
Chapter 5b (PowerPoint) Purpose Of Calorimeter Heat Capacity The symbol c stands for the specific heat. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.. Purpose Of Calorimeter Heat Capacity.
From klayjstec.blob.core.windows.net
How Calorimeter Function at Geneva Katzman blog Purpose Of Calorimeter Heat Capacity Calorimetry is used to measure amounts of heat transferred to. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences. Purpose Of Calorimeter Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Purpose Of Calorimeter Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate. Purpose Of Calorimeter Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Purpose Of Calorimeter Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state. Purpose Of Calorimeter Heat Capacity.
From www.slideserve.com
PPT CHEM 1011 PowerPoint Presentation, free download ID317228 Purpose Of Calorimeter Heat Capacity To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. The symbol c stands for the specific heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch. Purpose Of Calorimeter Heat Capacity.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Purpose Of Calorimeter Heat Capacity Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The symbol c stands for the specific heat. In this article, you will learn. Purpose Of Calorimeter Heat Capacity.
From www.youtube.com
Principle of Calorimetry YouTube Purpose Of Calorimeter Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms. Purpose Of Calorimeter Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Purpose Of Calorimeter Heat Capacity The symbol c stands for the specific heat. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. To measure the heat capacity of. Purpose Of Calorimeter Heat Capacity.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube Purpose Of Calorimeter Heat Capacity The symbol c stands for the specific heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. The heat capacity (c). Purpose Of Calorimeter Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Purpose Of Calorimeter Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calorimetry is used to measure amounts of heat transferred to.. Purpose Of Calorimeter Heat Capacity.
From 2012books.lardbucket.org
Calorimetry Purpose Of Calorimeter Heat Capacity Calorimetry is used to measure amounts of heat transferred to. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The symbol c stands for the specific heat. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard. Purpose Of Calorimeter Heat Capacity.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Purpose Of Calorimeter Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The symbol c stands for the specific heat. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. Calorimetry is. Purpose Of Calorimeter Heat Capacity.
From dxoinieui.blob.core.windows.net
Calorimeter Diagram Class 11 at Ollie Stringer blog Purpose Of Calorimeter Heat Capacity Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The symbol c stands for the specific heat. To measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is. Purpose Of Calorimeter Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Purpose Of Calorimeter Heat Capacity Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this article, you will learn about calorimetry and calorimeters,. Purpose Of Calorimeter Heat Capacity.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards Purpose Of Calorimeter Heat Capacity Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. One technique we can use to measure the amount of heat involved. Purpose Of Calorimeter Heat Capacity.