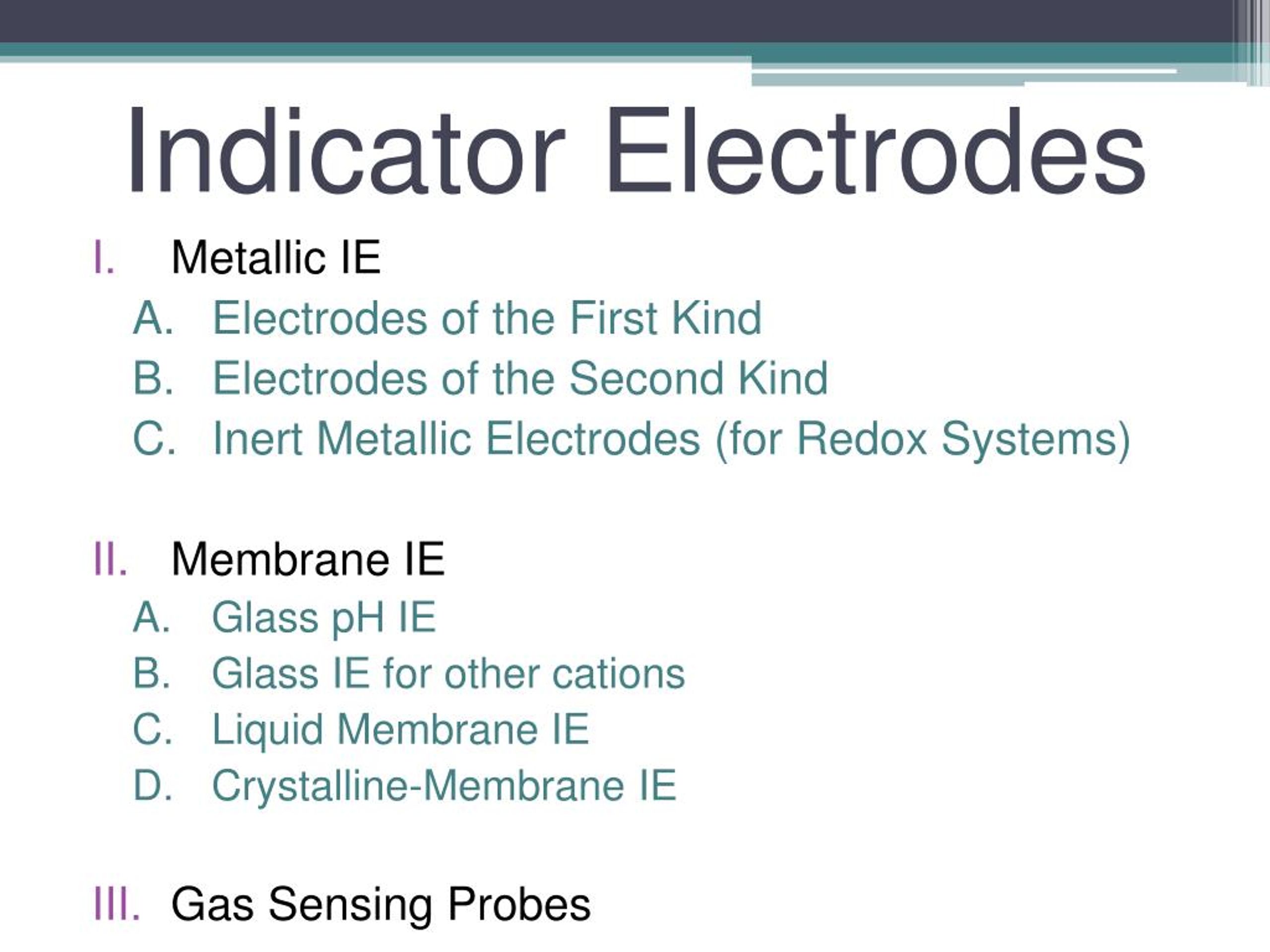
Indicator Electrode Definition . Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. The voltage measured is simply the difference between the potential at each electrode: The indicator electrode possesses some characteristic that allows it to. The potential of the indicator. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Learn about this topic in these articles:
from www.slideserve.com
The indicator electrode possesses some characteristic that allows it to. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. The potential of the indicator. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. The voltage measured is simply the difference between the potential at each electrode: An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. Learn about this topic in these articles: Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement.
PPT potentiometry PowerPoint Presentation, free download ID7435364
Indicator Electrode Definition An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Learn about this topic in these articles: The indicator electrode possesses some characteristic that allows it to. The voltage measured is simply the difference between the potential at each electrode: An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. The potential of the indicator.
From www.slideserve.com
PPT POTENTIOMETRY 8 th lecture PowerPoint Presentation, free download ID4526099 Indicator Electrode Definition The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. The indicator electrode possesses some characteristic that allows it to. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The potential of the indicator. An indicator electrode, also. Indicator Electrode Definition.
From www.youtube.com
Indicator electrodes metal electrodes glass electrode analysis unit 5 pharmacy Indicator Electrode Definition Learn about this topic in these articles: The voltage measured is simply the difference between the potential at each electrode: Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. The potential of the indicator. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. The ideal. Indicator Electrode Definition.
From www.scribd.com
Indicator Electrode Indicator Electrode Definition Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. An indicator. Indicator Electrode Definition.
From www.youtube.com
Chapter 15 Metal Indicator Electrodes CHM 214 142 YouTube Indicator Electrode Definition Learn about this topic in these articles: Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. The indicator electrode possesses some characteristic that allows it to. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. Metal indicator electrodes are systems that use. Indicator Electrode Definition.
From www.slideserve.com
PPT Electroanalytical chemistry PowerPoint Presentation, free download ID1287819 Indicator Electrode Definition The indicator electrode possesses some characteristic that allows it to. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Metal indicator electrodes are systems that use specific. Indicator Electrode Definition.
From www.studypool.com
SOLUTION Reference and indicator electrodes Studypool Indicator Electrode Definition An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. In potentiometry, those two electrodes are generally called the indicator electrode and the reference. Indicator Electrode Definition.
From www.brainkart.com
Metallic Indicator Electrodes Potentiometric Methods of Analysis Indicator Electrode Definition An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. Learn about this topic in these articles: The voltage measured is simply the difference between the potential at each electrode: The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present. Indicator Electrode Definition.
From www.slideserve.com
PPT potentiometry PowerPoint Presentation, free download ID7435364 Indicator Electrode Definition The voltage measured is simply the difference between the potential at each electrode: The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. Learn about this topic. Indicator Electrode Definition.
From www.slideserve.com
PPT Chapter 23 PowerPoint Presentation, free download ID151285 Indicator Electrode Definition The voltage measured is simply the difference between the potential at each electrode: Learn about this topic in these articles: Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. The indicator electrode possesses some characteristic that allows it to. Metal indicator electrodes are. Indicator Electrode Definition.
From www.slideserve.com
PPT Electrochemical methods of analysis PowerPoint Presentation, free download ID9563746 Indicator Electrode Definition Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. The indicator electrode possesses some characteristic that allows it to. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. An indicator electrode, also known as a working electrode, is an electrode that responds to changes. Indicator Electrode Definition.
From www.slideshare.net
1 Potentiometry Indicator Electrode Definition In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. The indicator electrode possesses some characteristic that allows it to. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. Learn about this topic in these articles: Metal indicator electrodes. Indicator Electrode Definition.
From www.metrohm.com
KF indicator electrode for automation Metrohm Indicator Electrode Definition The voltage measured is simply the difference between the potential at each electrode: Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. The ideal indicator electrode should react quickly to changes in ion concentration as. Indicator Electrode Definition.
From www.youtube.com
Metal Indicator Electrodes YouTube Indicator Electrode Definition The indicator electrode possesses some characteristic that allows it to. The potential of the indicator. The voltage measured is simply the difference between the potential at each electrode: In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. Potentiometric methods are based upon measurements. Indicator Electrode Definition.
From blog.hannaservice.eu
Choose the right Electrode for your Samples Hanna Instruments Africa Indicator Electrode Definition Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. The indicator electrode possesses some characteristic that allows it to. The voltage measured is simply the difference between the. Indicator Electrode Definition.
From www.youtube.com
Indicator Electrode Metal & Glass Indicator Electrode Potentiometry Analysis BP102T L Indicator Electrode Definition In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The voltage measured is simply the difference between the potential at. Indicator Electrode Definition.
From www.youtube.com
[Ch 2.4a] Metallic Indicator Electrodes YouTube Indicator Electrode Definition In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. The potential of the indicator. Learn about this topic in these articles: The voltage measured is simply the difference between the potential at each. Indicator Electrode Definition.
From studylib.net
Electrodes and Potentiometry Introduction 1.) Potentiometry Indicator Electrode Definition Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The indicator electrode possesses some. Indicator Electrode Definition.
From www.youtube.com
Glass Electrode I Indicator Electrode YouTube Indicator Electrode Definition The potential of the indicator. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. The indicator electrode possesses some characteristic that allows it to. An indicator electrode, also known as a working electrode, is an. Indicator Electrode Definition.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Indicator Electrode Definition In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. Metal indicator electrodes are systems that use specific metal electrodes as the indicator. Indicator Electrode Definition.
From www.slideserve.com
PPT ION SELECTIVE ELECTRODES PowerPoint Presentation ID2144993 Indicator Electrode Definition The indicator electrode possesses some characteristic that allows it to. The potential of the indicator. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration. Indicator Electrode Definition.
From treybramirezo.blob.core.windows.net
Glass Electrode Definition at treybramirezo blog Indicator Electrode Definition The potential of the indicator. An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. The voltage measured is simply the difference between the potential at each electrode: The indicator electrode possesses some characteristic that allows it to. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode.. Indicator Electrode Definition.
From nanohub.org
Resources Electrochemical Potentiometric Sensors for Environmental Monitoring Indicator Electrode Definition Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The potential of the indicator. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Learn about this topic in these articles: The voltage measured is simply the difference between the potential at each electrode: Electrochemical cell for a. Indicator Electrode Definition.
From en.ppt-online.org
Electroanalytical Chemistry online presentation Indicator Electrode Definition Learn about this topic in these articles: The indicator electrode possesses some characteristic that allows it to. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. The voltage measured is simply the difference between the potential at each electrode: Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The potential of. Indicator Electrode Definition.
From www.slideserve.com
PPT Electroanalytical methods PowerPoint Presentation, free download ID6136540 Indicator Electrode Definition Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. The voltage measured is simply the difference between the potential at each electrode: The indicator electrode possesses some characteristic that allows it to. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Metal indicator electrodes are systems that use specific metal electrodes. Indicator Electrode Definition.
From www.slideshare.net
INDICATOR ELECTRODE PPT Indicator Electrode Definition Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. The potential of the indicator. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Learn about this topic in these articles: The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample. Indicator Electrode Definition.
From www.slideserve.com
PPT potentiometry PowerPoint Presentation, free download ID7435364 Indicator Electrode Definition In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. Learn about this topic in these articles: The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in. Indicator Electrode Definition.
From www.difference.wiki
Indicator Electrode vs. Reference Electrode What’s the Difference? Indicator Electrode Definition An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The voltage measured is simply the difference between the potential at each. Indicator Electrode Definition.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation ID737892 Indicator Electrode Definition An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. Learn about this topic in these articles: The voltage measured is simply the difference between the potential at each electrode: Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. Potentiometric methods are based upon measurements of the. Indicator Electrode Definition.
From www.differencebetween.com
Difference Between Indicator Electrode and Reference Electrode Compare the Difference Between Indicator Electrode Definition Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. Learn about this topic in these articles: The voltage measured is simply the difference between the potential at each electrode: In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. An indicator electrode, also known as a working electrode, is an electrode that responds. Indicator Electrode Definition.
From www.scribd.com
Metal Indicator Electrodes PDF Analytical Chemistry Chemistry Indicator Electrode Definition Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The indicator electrode possesses some characteristic that allows it to. The potential of the indicator. The voltage measured is simply the difference between the potential at each electrode: Metal indicator electrodes are systems. Indicator Electrode Definition.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID4587685 Indicator Electrode Definition The voltage measured is simply the difference between the potential at each electrode: The indicator electrode possesses some characteristic that allows it to. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. Learn about this topic in these articles: Metal indicator electrodes. Indicator Electrode Definition.
From www.slideserve.com
PPT 146 Cells as chemical probes PowerPoint Presentation ID388826 Indicator Electrode Definition Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. Potentiometric methods are based upon measurements. Indicator Electrode Definition.
From www.youtube.com
Metallic Indicator electrodes_1 YouTube Indicator Electrode Definition The indicator electrode possesses some characteristic that allows it to. An indicator electrode, also known as a working electrode, is an electrode that responds to changes in the analyte concentration or. The potential of the indicator. The voltage measured is simply the difference between the potential at each electrode: The ideal indicator electrode should react quickly to changes in ion. Indicator Electrode Definition.
From www.askdifference.com
Indicator Electrode vs. Reference Electrode — What’s the Difference? Indicator Electrode Definition The indicator electrode possesses some characteristic that allows it to. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. The potential of the indicator. Learn about this topic in these articles: The voltage measured is. Indicator Electrode Definition.
From mavink.com
Electrode Classification Diagram Indicator Electrode Definition Metal indicator electrodes are systems that use specific metal electrodes as the indicator electrode in an electrochemical measurement. The potential of the indicator. Electrochemical cell for a potentiometric measurement with a metallic indicator electrode. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Learn about this topic in these articles: An indicator electrode, also. Indicator Electrode Definition.