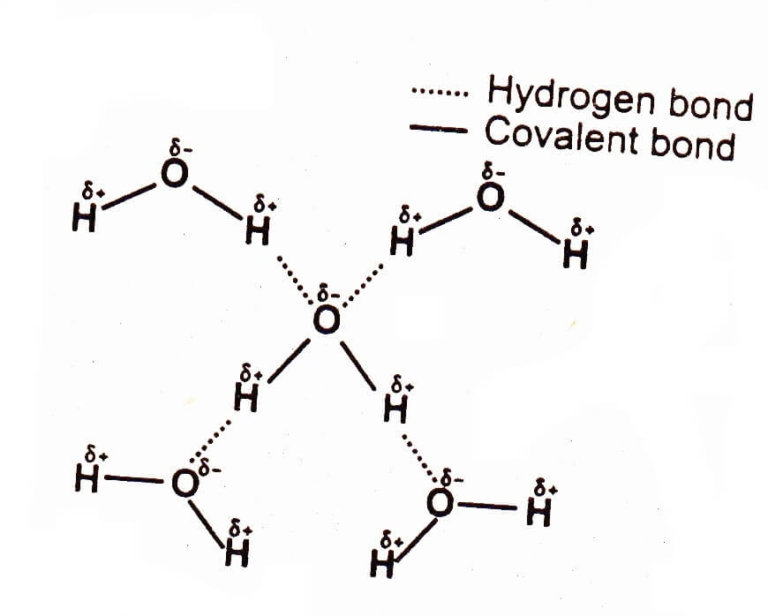
How Is Water Bonded To The Other Elements In The Molecule . Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Water has some unusual properties due to the hydrogen bonding between its molecules. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Describe the structure, such as it is, of. Describe the structure and shap of a water molecule. The atoms in a water. This is due to the water expanding as it is frozen. The density of ice is less than water. Identify the types of bonds within a water molecule and between. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar.
from chemistryskills.com
Describe the structure and shap of a water molecule. This is due to the water expanding as it is frozen. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Water has some unusual properties due to the hydrogen bonding between its molecules. Describe the structure, such as it is, of. The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The density of ice is less than water. Identify the types of bonds within a water molecule and between.
Hydrogen Bonding Chemistry Skills
How Is Water Bonded To The Other Elements In The Molecule The density of ice is less than water. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Describe the structure and shap of a water molecule. Identify the types of bonds within a water molecule and between. Water has some unusual properties due to the hydrogen bonding between its molecules. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The density of ice is less than water. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The atoms in a water. Describe the structure, such as it is, of. The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. This is due to the water expanding as it is frozen.
From klaszcmjb.blob.core.windows.net
How Is Water Bonded Together at Anthony Faison blog How Is Water Bonded To The Other Elements In The Molecule This is due to the water expanding as it is frozen. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Identify the types of bonds within a water molecule and between. Explain what is meant by hydrogen bonding and the. How Is Water Bonded To The Other Elements In The Molecule.
From www.youtube.com
Structure Of Water Molecule Chemistry Of Water Properties Of Water How Is Water Bonded To The Other Elements In The Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Identify the types of bonds within a water molecule and between. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Describe the structure, such as it is, of. Because of the higher electronegativity of the oxygen atom,. How Is Water Bonded To The Other Elements In The Molecule.
From cejomfvi.blob.core.windows.net
Importance Of Hydrogen Bonds at Katherine Perez blog How Is Water Bonded To The Other Elements In The Molecule Water has some unusual properties due to the hydrogen bonding between its molecules. The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Explain what is. How Is Water Bonded To The Other Elements In The Molecule.
From www.pinterest.com.au
Water has both a hydrogen bond and a polar covalent bond. Hydrogen How Is Water Bonded To The Other Elements In The Molecule Water has some unusual properties due to the hydrogen bonding between its molecules. The density of ice is less than water. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a simple molecule consisting of one oxygen atom. How Is Water Bonded To The Other Elements In The Molecule.
From www.pinterest.com
illustration of biochemistry, Water molecule consists of two hydrogen How Is Water Bonded To The Other Elements In The Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. This is due to the water expanding as it is frozen. Water has some unusual properties due to the hydrogen bonding between its molecules. Describe the structure, such as it is, of. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The atoms. How Is Water Bonded To The Other Elements In The Molecule.
From www.britannica.com
Water Definition, Chemical Formula, Structure, Molecule, & Facts How Is Water Bonded To The Other Elements In The Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The density of ice is less than water. Because of the higher electronegativity of the oxygen atom, the bonds are polar. This is due to the water expanding as it is frozen. Describe the structure, such as it is, of. Explain what is meant. How Is Water Bonded To The Other Elements In The Molecule.
From drmchemistrytutor.com
Hydrogen Bonding in water Dr. M. Chemistry Tutor How Is Water Bonded To The Other Elements In The Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. The atoms in a water. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Identify the types of bonds within a water molecule and between. This is due to the water expanding as it is frozen. Describe the structure, such. How Is Water Bonded To The Other Elements In The Molecule.
From courses.lumenlearning.com
Intermolecular Forces Chemistry for Majors How Is Water Bonded To The Other Elements In The Molecule The atoms in a water. This is due to the water expanding as it is frozen. Identify the types of bonds within a water molecule and between. Water has some unusual properties due to the hydrogen bonding between its molecules. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The density of ice is less than. How Is Water Bonded To The Other Elements In The Molecule.
From www.coursehero.com
[Solved] Label the bonds and components of this water molecule How Is Water Bonded To The Other Elements In The Molecule Describe the structure, such as it is, of. The atoms in a water. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Identify the types of bonds within a water molecule and between. The chemical formula for water is h. How Is Water Bonded To The Other Elements In The Molecule.
From www.britannica.com
chemical bonding Definition, Types, & Examples Britannica How Is Water Bonded To The Other Elements In The Molecule Water has some unusual properties due to the hydrogen bonding between its molecules. Describe the structure and shap of a water molecule. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The density of ice is less than water. This. How Is Water Bonded To The Other Elements In The Molecule.
From www.alamy.com
Reaction of Hydrogen and Oxygen in New compounds. Water molecule that How Is Water Bonded To The Other Elements In The Molecule Describe the structure, such as it is, of. This is due to the water expanding as it is frozen. The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. Water is a simple molecule consisting of one oxygen atom bonded to two. How Is Water Bonded To The Other Elements In The Molecule.
From chamotgallery.com
Hydrogen Bonding Definition, Types, Effects and Properties (2022) How Is Water Bonded To The Other Elements In The Molecule The atoms in a water. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Because of the higher electronegativity of the oxygen atom, the bonds are polar. This is due to the water expanding as it is frozen. Describe the. How Is Water Bonded To The Other Elements In The Molecule.
From www.slideserve.com
PPT Water Chemistry & Properties of Water PowerPoint Presentation How Is Water Bonded To The Other Elements In The Molecule Describe the structure, such as it is, of. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Describe the structure and shap of a water molecule. This is due to the water expanding as it is frozen.. How Is Water Bonded To The Other Elements In The Molecule.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure How Is Water Bonded To The Other Elements In The Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The atoms in a water. The density of ice is less than water. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Identify the types of bonds within a water molecule and between. Because of the higher. How Is Water Bonded To The Other Elements In The Molecule.
From answerdbmaligner.z21.web.core.windows.net
Why Are Hydrogen Bonds Important In Water How Is Water Bonded To The Other Elements In The Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. This is due to the water expanding as it is frozen. The. How Is Water Bonded To The Other Elements In The Molecule.
From mavink.com
Water Molecules Hydrogen Bonding How Is Water Bonded To The Other Elements In The Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. The atoms in a water. The density of ice is less than water. Describe the structure, such as it is, of. The chemical formula for water is h. How Is Water Bonded To The Other Elements In The Molecule.
From taylorsciencegeeks.weebly.com
The Water Molecule Science News How Is Water Bonded To The Other Elements In The Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The atoms in a water. The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one. How Is Water Bonded To The Other Elements In The Molecule.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic How Is Water Bonded To The Other Elements In The Molecule Describe the structure, such as it is, of. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The density of ice is less than water. The atoms in a water. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The chemical formula for water is h 2 o which indicates that a. How Is Water Bonded To The Other Elements In The Molecule.
From openoregon.pressbooks.pub
Water Principles of Biology How Is Water Bonded To The Other Elements In The Molecule Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Identify the types of bonds within a water molecule and between. Water has some unusual properties due to the hydrogen bonding between its molecules. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Describe the structure and shap of a. How Is Water Bonded To The Other Elements In The Molecule.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID How Is Water Bonded To The Other Elements In The Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The atoms in a water. This is due to the water expanding as it is frozen. The chemical formula for water is h 2 o which indicates that a single molecule. How Is Water Bonded To The Other Elements In The Molecule.
From biostudizz.weebly.com
WATER CHEMISTRY to Bio Stud... How Is Water Bonded To The Other Elements In The Molecule The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Describe the structure and shap of a water molecule. The atoms in a water. Water has. How Is Water Bonded To The Other Elements In The Molecule.
From quizlet.com
hydrogen bond between water molecules Diagram Quizlet How Is Water Bonded To The Other Elements In The Molecule Identify the types of bonds within a water molecule and between. Water has some unusual properties due to the hydrogen bonding between its molecules. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Describe the structure and. How Is Water Bonded To The Other Elements In The Molecule.
From www.science-revision.co.uk
Covalent bonding How Is Water Bonded To The Other Elements In The Molecule Describe the structure, such as it is, of. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The atoms in a. How Is Water Bonded To The Other Elements In The Molecule.
From owlcation.com
Primary and Secondary Bonds Owlcation How Is Water Bonded To The Other Elements In The Molecule Water has some unusual properties due to the hydrogen bonding between its molecules. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Describe the structure, such as it is, of. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Identify the types of bonds within a. How Is Water Bonded To The Other Elements In The Molecule.
From essenceofwater.org
Structure of Water Essence of Water Essence of Water How Is Water Bonded To The Other Elements In The Molecule Describe the structure and shap of a water molecule. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The atoms in a water. This is due to the water expanding as it is frozen. Describe the structure,. How Is Water Bonded To The Other Elements In The Molecule.
From www.snexplores.org
Explainer What are chemical bonds? How Is Water Bonded To The Other Elements In The Molecule The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. This is due to the water expanding as it is frozen. Water has some unusual properties due to the hydrogen bonding between its molecules. Describe the structure, such as it is, of.. How Is Water Bonded To The Other Elements In The Molecule.
From scienceatyourdoorstep.com
Types of Atoms Science at Your Doorstep How Is Water Bonded To The Other Elements In The Molecule Water has some unusual properties due to the hydrogen bonding between its molecules. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The density. How Is Water Bonded To The Other Elements In The Molecule.
From www.alamy.com
Diagram to illustrate covalent bonding in water with a fully labelled How Is Water Bonded To The Other Elements In The Molecule Describe the structure and shap of a water molecule. Identify the types of bonds within a water molecule and between. The atoms in a water. The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. Describe the structure, such as it is,. How Is Water Bonded To The Other Elements In The Molecule.
From www.sciencephoto.com
Bond formation in water molecule Stock Image C028/6477 Science How Is Water Bonded To The Other Elements In The Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. The atoms in a water. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Describe the structure, such as it is, of. Water has some unusual properties due to the hydrogen bonding between its molecules. Identify the types of bonds. How Is Water Bonded To The Other Elements In The Molecule.
From schematicfixtrysted.z22.web.core.windows.net
Water Molecule With Labels How Is Water Bonded To The Other Elements In The Molecule The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen atoms and one oxygen atom. Water has some unusual properties due to the hydrogen bonding between its molecules. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Because of the higher electronegativity of. How Is Water Bonded To The Other Elements In The Molecule.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Water and pH How Is Water Bonded To The Other Elements In The Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. Identify the types of bonds within a water molecule and between. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The atoms in a water. The chemical formula for water is h 2 o which indicates that a single molecule. How Is Water Bonded To The Other Elements In The Molecule.
From chemistryskills.com
Hydrogen Bonding Chemistry Skills How Is Water Bonded To The Other Elements In The Molecule Describe the structure, such as it is, of. Identify the types of bonds within a water molecule and between. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Describe the structure and shap of a water molecule. The chemical formula for water is h 2 o which indicates that a single molecule of water is made. How Is Water Bonded To The Other Elements In The Molecule.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. How Is Water Bonded To The Other Elements In The Molecule Describe the structure, such as it is, of. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Identify the types of bonds within a water molecule and between. This is due to the water expanding as it is frozen. The chemical formula for water is h 2 o which indicates that a single molecule of water. How Is Water Bonded To The Other Elements In The Molecule.
From www2.victoriacollege.edu
polar covalent bonds of water How Is Water Bonded To The Other Elements In The Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. Identify the types of bonds within a water molecule and between. Water has some unusual properties due to the hydrogen bonding between its molecules. The chemical formula for water is h 2 o which indicates that a single molecule of water is made up of two hydrogen. How Is Water Bonded To The Other Elements In The Molecule.
From paleolimbot.github.io
Chapter 5 Minerals Physical Geology How Is Water Bonded To The Other Elements In The Molecule Identify the types of bonds within a water molecule and between. Water has some unusual properties due to the hydrogen bonding between its molecules. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The atoms in a water. Describe the structure, such as it is, of. The chemical formula for water is h 2 o which. How Is Water Bonded To The Other Elements In The Molecule.