Titration Labster Answer Key . What is the concentration of naoh? A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? 21.6 ml of naoh was required to. Titration lab answer key titration lab: If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid solution? Exploring beyond the basics chapter 6: Determine the concentration of hcl. What is the end point of a titration? Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. A volume of 30.0 ml of 0.25. 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh.
from www.studocu.com
If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid solution? 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. 21.6 ml of naoh was required to. Exploring beyond the basics chapter 6: What is the end point of a titration? Titration lab answer key titration lab: If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. A volume of 30.0 ml of 0.25. 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh.
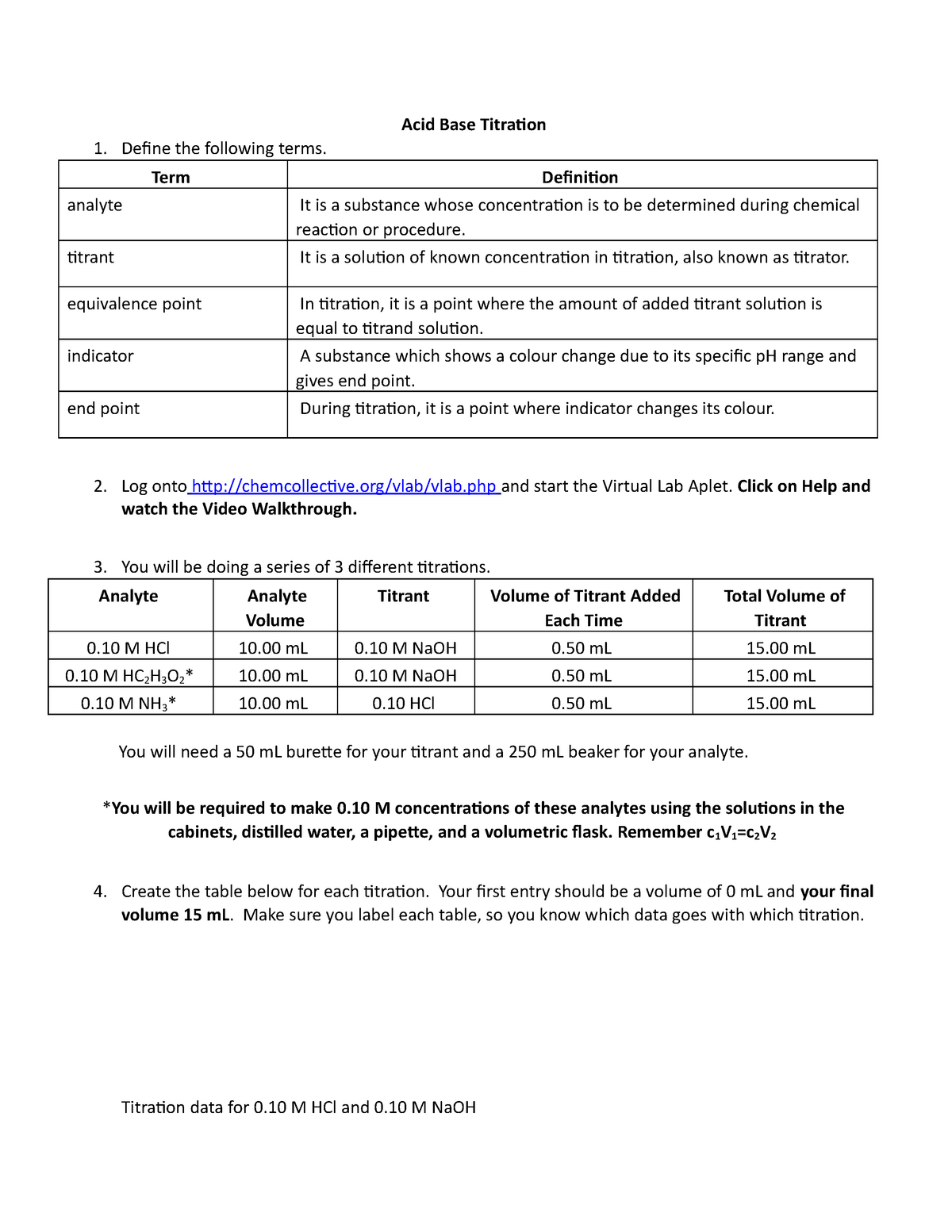
Acid Base Titration Virtual Lab Nov 2020 Acid Base Titraion Deine the
Titration Labster Answer Key Exploring beyond the basics chapter 6: If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid solution? Determine the concentration of hcl. Exploring beyond the basics chapter 6: 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? What is the concentration of naoh? 21.6 ml of naoh was required to. Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. Titration lab answer key titration lab: A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. A volume of 30.0 ml of 0.25. What is the end point of a titration? 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh.
From www.studocu.com
Titrationlevel4labnotebook Titration level Bethany. S Aims In Titration Labster Answer Key If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? What is the concentration of naoh? Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. 21.6 ml of naoh was required to. 14.14ml of 0.125m (mol/l). Titration Labster Answer Key.
From academguide.com
Unlocking the Secrets of Titration Student Exploration Answer Key Revealed Titration Labster Answer Key 21.6 ml of naoh was required to. Exploring beyond the basics chapter 6: Titration lab answer key titration lab: What is the end point of a titration? Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. 14.14ml of 0.125m (mol/l) hcl is used to. Titration Labster Answer Key.
From www.labster.com
Virtual Lab Titration Virtual Lab Labster Titration Labster Answer Key What is the end point of a titration? Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. Determine the concentration of hcl. 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. Exploring beyond the basics. Titration Labster Answer Key.
From worksheetzonegibson55.z21.web.core.windows.net
Titrations Practice Worksheet Answers Titration Labster Answer Key What is the concentration of naoh? Determine the concentration of hcl. What is the end point of a titration? 21.6 ml of naoh was required to. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? Titration lab answer key titration lab: A titration was performed using 1.00 m naoh as the. Titration Labster Answer Key.
From www.studocu.com
Practice Problems Week 6 p H and Titrations With Answer Key Study Titration Labster Answer Key 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. Titration Labster Answer Key.
From www.studocu.com
Titration Gizmo Student Exploration Titration Activity A Acids and Titration Labster Answer Key Exploring beyond the basics chapter 6: 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. 21.6 ml of naoh was required to. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? What is the end point of a titration? Study with quizlet. Titration Labster Answer Key.
From www.chegg.com
Solved Week 6 Lab Titration Concentration of Vinegar Titration Labster Answer Key Determine the concentration of hcl. What is the end point of a titration? 21.6 ml of naoh was required to. A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. Titration lab. Titration Labster Answer Key.
From theschoolusa.com
Key answers for a titration worksheet Titration Labster Answer Key 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. A volume of 30.0 ml of 0.25. What is the end point of a titration? If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? What is the concentration of naoh? A titration was. Titration Labster Answer Key.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Titration Labster Answer Key What is the concentration of naoh? 21.6 ml of naoh was required to. A volume of 30.0 ml of 0.25. What is the end point of a titration? Titration lab answer key titration lab: A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. Study with quizlet and memorize flashcards. Titration Labster Answer Key.
From www.docsity.com
Acid base titration lab answers Docsity Titration Labster Answer Key 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. Determine the concentration of hcl. What is the end point of a titration? A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. Study with quizlet and memorize flashcards containing terms like. Titration Labster Answer Key.
From www.studocu.com
W5 Titration Curves KEY 5 Titration Curves ANSWER KEY A pH Titration Labster Answer Key Exploring beyond the basics chapter 6: A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. What is the concentration of naoh? What is the end point of a titration? 21.6 ml of naoh was required to. Titration lab answer key titration lab: Study with quizlet and memorize flashcards containing. Titration Labster Answer Key.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Titration Labster Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid solution? A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. Exploring beyond the basics chapter 6: 4) can i titrate a solution of unknown concentration with another. Titration Labster Answer Key.
From wks.udlvirtual.edu.pe
Acid Base Titration Practice Worksheet Worksheets Printable Free Titration Labster Answer Key 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. Exploring beyond the basics chapter 6: Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. 4) can i titrate a solution of unknown concentration with another. Titration Labster Answer Key.
From worksheetzonegibson55.z21.web.core.windows.net
Titration Worksheet Answer Key Titration Labster Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid solution? Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. Determine the concentration of hcl. A volume of 30.0 ml of 0.25.. Titration Labster Answer Key.
From www.studocu.com
Acid Base Titration Virtual Lab Nov 2020 Acid Base Titraion Deine the Titration Labster Answer Key What is the end point of a titration? Exploring beyond the basics chapter 6: 21.6 ml of naoh was required to. A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. 4). Titration Labster Answer Key.
From qwivy.com
Exam Gizmos Student Exploration Titration Answer Key Gizmos Student Titration Labster Answer Key Exploring beyond the basics chapter 6: Determine the concentration of hcl. 21.6 ml of naoh was required to. A volume of 30.0 ml of 0.25. A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. What is the concentration of naoh? Study with quizlet and memorize flashcards containing terms like. Titration Labster Answer Key.
From www.studypool.com
SOLUTION Titrations questions with answers Studypool Titration Labster Answer Key 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. What is the concentration of naoh? Titration lab answer key titration lab: 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. If the final burette reading is 28.45 and the initial. Titration Labster Answer Key.
From www.scribd.com
titration_answer_keys PDF Titration Labster Answer Key A volume of 30.0 ml of 0.25. What is the end point of a titration? Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. What is the concentration of naoh? Titration lab answer key titration lab: Exploring beyond the basics chapter 6: A titration. Titration Labster Answer Key.
From myans.bhantedhammika.net
Acids And Bases Titration Worksheet Answer Key Titration Labster Answer Key Exploring beyond the basics chapter 6: A volume of 30.0 ml of 0.25. A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. If 15.0 ml of 0.50 m naoh is used. Titration Labster Answer Key.
From yazminewadiaz.blogspot.com
Acid Base Titration Lab Report Answers YazminewaDiaz Titration Labster Answer Key A volume of 30.0 ml of 0.25. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. 4) can i titrate a solution of unknown concentration with another solution of unknown concentration. Titration Labster Answer Key.
From www.studypool.com
SOLUTION Titration Student Exploration Worksheet Studypool Titration Labster Answer Key 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. Titration lab answer key titration lab: What is the end point of a titration? 21.6 ml of naoh was required to. 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. Study. Titration Labster Answer Key.
From www.studypool.com
SOLUTION Titration Student Exploration Worksheet Studypool Titration Labster Answer Key A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. Determine the concentration of hcl. What is the end point of a titration? Study with quizlet and memorize flashcards containing terms like. Titration Labster Answer Key.
From studyfinder.org
Mastering Titrations Unlocking the Student Exploration Gizmo with Titration Labster Answer Key If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. 21.6 ml of naoh was required to. Determine the concentration of hcl. If 15.0 ml of 0.50 m naoh is used to. Titration Labster Answer Key.
From www.labster.com
Virtual Lab Titration Virtual Lab Labster Titration Labster Answer Key 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. What is the end point of a titration? A volume of 30.0 ml of 0.25. A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. Exploring beyond the basics chapter. Titration Labster Answer Key.
From gioufpirl.blob.core.windows.net
Titration Lab Procedure High School at Estelle Hart blog Titration Labster Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid solution? A volume of 30.0 ml of 0.25. 21.6 ml of naoh was required to. 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. Titration lab answer. Titration Labster Answer Key.
From gioiamhcx.blob.core.windows.net
Titration Chemistry Questions at Butterfield blog Titration Labster Answer Key What is the concentration of naoh? Titration lab answer key titration lab: Determine the concentration of hcl. Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. Exploring beyond the basics chapter 6: A titration was performed using 1.00 m naoh as the titrant and. Titration Labster Answer Key.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Titration Labster Answer Key 21.6 ml of naoh was required to. 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? A volume of 30.0 ml of 0.25. Titration lab answer key titration lab: A titration. Titration Labster Answer Key.
From www.studocu.com
Titration (Gizmo) Lab 201 Titration (Gizmo) Lab Vocabulary acid Titration Labster Answer Key 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. Titration lab answer key titration lab: What is the concentration of naoh? Determine the concentration of hcl. 21.6 ml of naoh was required to. A volume of 30.0 ml of 0.25. 4) can i titrate a solution of unknown concentration with another. Titration Labster Answer Key.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Titration Labster Answer Key Titration lab answer key titration lab: Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. A volume of 30.0 ml of 0.25. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? Determine the concentration of. Titration Labster Answer Key.
From www.scribd.com
GCSE Chemistry Titrations Questions With Answers PDF Chemistry Titration Labster Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid solution? Determine the concentration of hcl. If the final burette reading is 28.45 and the initial burette reading is 5.60, what is the titer? A titration was performed using 1.00 m naoh as the titrant and an unknown. Titration Labster Answer Key.
From www.scribd.com
Titration Calculation Answer Key PDF Ph Chemistry Titration Labster Answer Key Titration lab answer key titration lab: 14.14ml of 0.125m (mol/l) hcl is used to reach the endpoint of a titration with 10.00ml of naoh. A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what. Titration Labster Answer Key.
From exyrvephg.blob.core.windows.net
Titration Calculations Worksheet Gcse at Margo Davis blog Titration Labster Answer Key A volume of 30.0 ml of 0.25. 21.6 ml of naoh was required to. Exploring beyond the basics chapter 6: Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. Titration lab answer key titration lab: 14.14ml of 0.125m (mol/l) hcl is used to reach. Titration Labster Answer Key.
From www.studocu.com
Titration+Answer+Key 2019 Titration Answer Key Vocabulary acid Titration Labster Answer Key 4) can i titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful. A volume of 30.0 ml of 0.25. Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. What is the concentration of naoh? 14.14ml of. Titration Labster Answer Key.
From www.studypool.com
SOLUTION Answers labster Studypool Titration Labster Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid solution? A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. Study with quizlet and memorize flashcards containing terms like what do you think we need to do. Titration Labster Answer Key.
From www.scribd.com
(Answer Key) Practice Set Titration and AcidBase Titration PDF Titration Labster Answer Key Determine the concentration of hcl. Study with quizlet and memorize flashcards containing terms like what do you think we need to do to help the lake ecosystem?, today we are. A titration was performed using 1.00 m naoh as the titrant and an unknown concentration of h2so4 as the analyte. 4) can i titrate a solution of unknown concentration with. Titration Labster Answer Key.