Why Q Is Not Equal To 0 . The quick answer is $\delta u \neq 0$. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. The process is not isentropic, as. In the special case where you are dealing with ideal gas. The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. I understand that during isothermal reactions, delta u=0. I also understand that if work is being done, then w cannot be zero hence q. Because \(q\) < k, the reaction is not at equilibrium and proceeds to. To further understand the relationship between heat flow (q) and the resulting change in internal energy. Let's look at some details. Enthalpy as a composite function.
from www.youtube.com
The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). I understand that during isothermal reactions, delta u=0. To further understand the relationship between heat flow (q) and the resulting change in internal energy. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. I also understand that if work is being done, then w cannot be zero hence q. The quick answer is $\delta u \neq 0$. In the special case where you are dealing with ideal gas. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. Enthalpy as a composite function. Because \(q\) < k, the reaction is not at equilibrium and proceeds to.
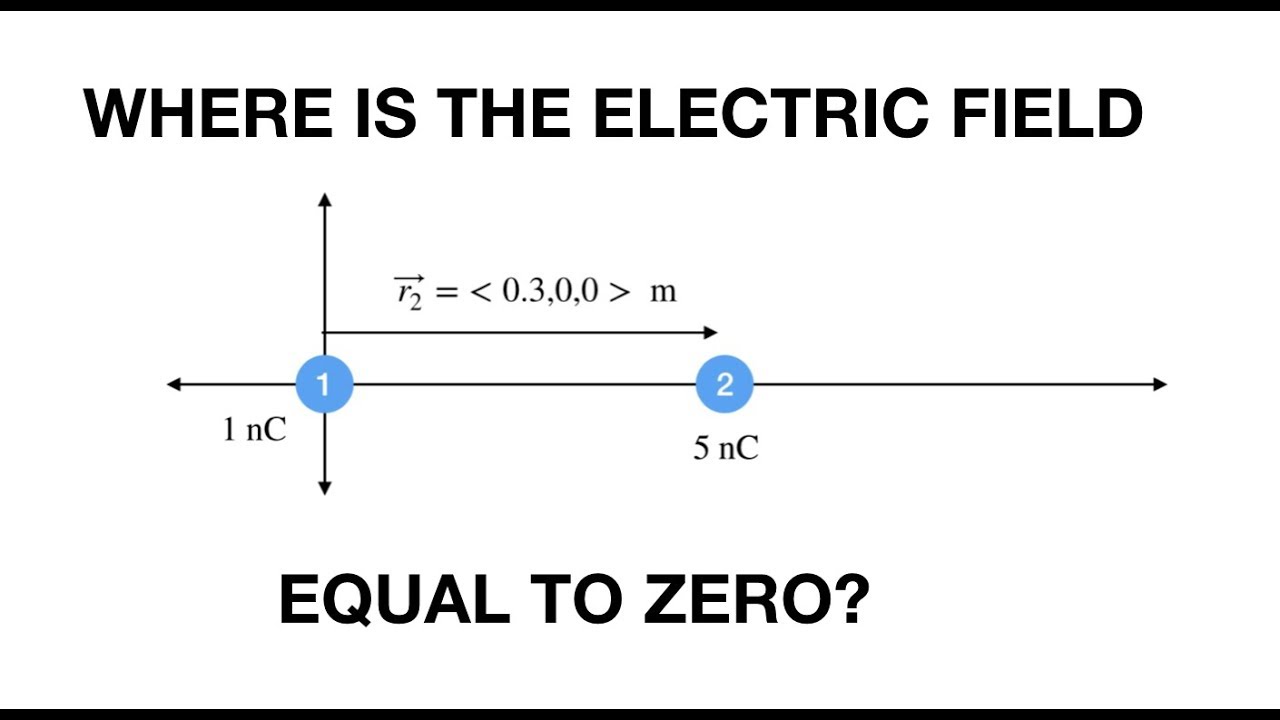
Where is the electric field equal to zero? YouTube
Why Q Is Not Equal To 0 Let's look at some details. The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). Enthalpy as a composite function. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. To further understand the relationship between heat flow (q) and the resulting change in internal energy. Because \(q\) < k, the reaction is not at equilibrium and proceeds to. Let's look at some details. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. I also understand that if work is being done, then w cannot be zero hence q. I understand that during isothermal reactions, delta u=0. The quick answer is $\delta u \neq 0$. In the special case where you are dealing with ideal gas. The process is not isentropic, as.
From brainly.in
Express 0.47 bar in the form of p/q.where p and q are integers!!and q Why Q Is Not Equal To 0 Because \(q\) < k, the reaction is not at equilibrium and proceeds to. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. Let's look at some details. The quick answer is $\delta u \neq 0$. I also understand that if work is being done, then w cannot be zero hence. Why Q Is Not Equal To 0.
From www.youtube.com
Show that 0.3333... Can be expressed in the form p/q . Where p and q Why Q Is Not Equal To 0 Enthalpy as a composite function. I understand that during isothermal reactions, delta u=0. In the special case where you are dealing with ideal gas. The quick answer is $\delta u \neq 0$. I also understand that if work is being done, then w cannot be zero hence q. To further understand the relationship between heat flow (q) and the resulting. Why Q Is Not Equal To 0.
From brainly.in
Express 0.3999... in the form p/q where p and q are integers and q ≠ 0 Why Q Is Not Equal To 0 In the special case where you are dealing with ideal gas. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. Enthalpy as a composite function. The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). Let's look at some details. Because. Why Q Is Not Equal To 0.
From brainly.in
Urgent pls help Express 0.327272727.... in form of p/q where p and q Why Q Is Not Equal To 0 To further understand the relationship between heat flow (q) and the resulting change in internal energy. Let's look at some details. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$. Why Q Is Not Equal To 0.
From brainly.in
express 0.2(27)bar in the form of p/q where p and q are integers and q Why Q Is Not Equal To 0 I understand that during isothermal reactions, delta u=0. The process is not isentropic, as. The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). The quick answer is $\delta u \neq 0$. Because \(q\) < k, the reaction is not at equilibrium and proceeds to. The internal energy deltau only depends on temperature. Why Q Is Not Equal To 0.
From brainly.in
Express 2.015bar in the p/q form, where p and q are integers and q Why Q Is Not Equal To 0 To further understand the relationship between heat flow (q) and the resulting change in internal energy. The quick answer is $\delta u \neq 0$. The process is not isentropic, as. I understand that during isothermal reactions, delta u=0. Enthalpy as a composite function. I also understand that if work is being done, then w cannot be zero hence q. The. Why Q Is Not Equal To 0.
From www.youtube.com
Why Does A Number Raised To Zero Equal One? YouTube Why Q Is Not Equal To 0 The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). Let's look at some details. I also understand that if work is being done, then w cannot be zero hence q. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. The. Why Q Is Not Equal To 0.
From brainly.in
express 0.8181.... in the form of p/q where p and q are integers and q Why Q Is Not Equal To 0 To further understand the relationship between heat flow (q) and the resulting change in internal energy. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). Because \(q\) < k, the reaction is not. Why Q Is Not Equal To 0.
From brainly.in
show that 0.2727.....can be expressed in the form p/q ,p, q £ Z,q is Why Q Is Not Equal To 0 Let's look at some details. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. To further understand the relationship between heat flow (q) and the resulting change in internal energy. The process is not isentropic, as. Enthalpy as a composite function. I understand that during isothermal reactions, delta. Why Q Is Not Equal To 0.
From brainly.in
Express 0.54bar in the form p/q where q not equal to 0 Brainly.in Why Q Is Not Equal To 0 In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. The quick answer is $\delta u \neq 0$. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. Let's look at some details. I also understand that if work is. Why Q Is Not Equal To 0.
From brainly.in
The zeroes of the quadratic polynomial x2 +kx+k ,k not equal to 0 Why Q Is Not Equal To 0 I understand that during isothermal reactions, delta u=0. The process is not isentropic, as. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. Because \(q\) < k, the reaction. Why Q Is Not Equal To 0.
From www.youtube.com
Why 0.999... is Equal to 1 YouTube Why Q Is Not Equal To 0 The process is not isentropic, as. The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). Because \(q\) < k, the reaction is not at equilibrium and proceeds to. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. In the special case where. Why Q Is Not Equal To 0.
From www.youtube.com
express the following in the form p/q where p and q are integers and q Why Q Is Not Equal To 0 Enthalpy as a composite function. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. The process is not isentropic, as. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. I also understand that if work is being done,. Why Q Is Not Equal To 0.
From brainly.in
Express 23.43bar in p by q where p, q are integers and q not equal to 0 Why Q Is Not Equal To 0 Let's look at some details. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. The process is not isentropic, as. In the special case where you are dealing with ideal gas. Because \(q\) < k, the reaction is not at equilibrium and proceeds to. The quick answer is. Why Q Is Not Equal To 0.
From brainly.in
express 0.001 bar in the form of p/q where p/q are integers and q is Why Q Is Not Equal To 0 To further understand the relationship between heat flow (q) and the resulting change in internal energy. Enthalpy as a composite function. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. Let's look at some details. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity. Why Q Is Not Equal To 0.
From brainly.in
Show that 1.27 bar on 27 can be expressed in the form of p/q, where p/q Why Q Is Not Equal To 0 I understand that during isothermal reactions, delta u=0. The process is not isentropic, as. In the special case where you are dealing with ideal gas. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. Let's look at some details. I also understand that if work is being done, then w. Why Q Is Not Equal To 0.
From www.toppr.com
"Q.1 Express ( 1.4191919 ldots ) in the form of ( frac { 1 } { 9 Why Q Is Not Equal To 0 The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. Let's look at some details. In the special case where you are dealing with ideal gas. To further understand the relationship between heat flow (q) and the resulting change in internal energy. The quick answer is $\delta u \neq 0$. I. Why Q Is Not Equal To 0.
From www.meritnation.com
Express the following in pq form where p and q are integers and q =not Why Q Is Not Equal To 0 Let's look at some details. I understand that during isothermal reactions, delta u=0. Enthalpy as a composite function. The process is not isentropic, as. To further understand the relationship between heat flow (q) and the resulting change in internal energy. The quick answer is $\delta u \neq 0$. The internal energy deltau only depends on temperature for ideal gases, so. Why Q Is Not Equal To 0.
From brainly.in
Is zero a rational number? Can you write it in the form p/q where p and Why Q Is Not Equal To 0 In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. To further understand the relationship between heat flow (q) and the resulting change in internal energy. Because \(q\) < k, the reaction is not at equilibrium and proceeds to. The internal energy deltau only depends on temperature for ideal. Why Q Is Not Equal To 0.
From brainly.in
2. Show that 0.2353535... = 0.235 can be expressed in the form, where p Why Q Is Not Equal To 0 In the special case where you are dealing with ideal gas. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. The quick answer is $\delta u \neq 0$. Because \(q\) < k, the reaction is not at equilibrium and proceeds to. Let's look at some details. I understand. Why Q Is Not Equal To 0.
From brainly.in
express 0.47(bar on 7) in the form of p/q where p and q are integers Why Q Is Not Equal To 0 Because \(q\) < k, the reaction is not at equilibrium and proceeds to. In the special case where you are dealing with ideal gas. To further understand the relationship between heat flow (q) and the resulting change in internal energy. I understand that during isothermal reactions, delta u=0. The internal energy deltau only depends on temperature for ideal gases, so. Why Q Is Not Equal To 0.
From brainly.in
Express 18.48 ( 48 bar) in the form of p/q where p and q are integers q Why Q Is Not Equal To 0 In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). I also understand that if work is being done, then w cannot be zero hence q. To further understand the relationship between. Why Q Is Not Equal To 0.
From brainly.in
Express 0.8 bar in the form p\q where pand are integer and q not equal Why Q Is Not Equal To 0 To further understand the relationship between heat flow (q) and the resulting change in internal energy. I also understand that if work is being done, then w cannot be zero hence q. I understand that during isothermal reactions, delta u=0. The process is not isentropic, as. Enthalpy as a composite function. The internal energy deltau only depends on temperature for. Why Q Is Not Equal To 0.
From brainly.in
Write the following terminating decimals in the form of p/q,q not equal Why Q Is Not Equal To 0 I also understand that if work is being done, then w cannot be zero hence q. To further understand the relationship between heat flow (q) and the resulting change in internal energy. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. The \(q\) value, 0.436, is less than the given. Why Q Is Not Equal To 0.
From brainly.in
Show that 0.47777777.... Can be expressed in the form p/q ,where p and Why Q Is Not Equal To 0 In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. The process is not isentropic, as. Enthalpy as a composite function. I also understand that if work is being done, then w cannot be zero hence q. The \(q\) value, 0.436, is less than the given \(k\) value of. Why Q Is Not Equal To 0.
From brainly.in
Express the following in the form of p and q , Where p and q are Why Q Is Not Equal To 0 Let's look at some details. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. I also understand that if work is being done, then w cannot be zero hence q. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$. Why Q Is Not Equal To 0.
From brainly.in
express 0.4323232....... in the form p/q, where p and q are integers Why Q Is Not Equal To 0 I also understand that if work is being done, then w cannot be zero hence q. The quick answer is $\delta u \neq 0$. To further understand the relationship between heat flow (q) and the resulting change in internal energy. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$. Why Q Is Not Equal To 0.
From cameramath.com
[Solved] 8. Construct Arguments Can you divide 14 shirts into 2 equal Why Q Is Not Equal To 0 Enthalpy as a composite function. To further understand the relationship between heat flow (q) and the resulting change in internal energy. Let's look at some details. In the special case where you are dealing with ideal gas. I also understand that if work is being done, then w cannot be zero hence q. Because \(q\) < k, the reaction is. Why Q Is Not Equal To 0.
From www.youtube.com
Where is the electric field equal to zero? YouTube Why Q Is Not Equal To 0 The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). I also understand that if work is being done, then w cannot be zero hence q. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. Let's look at some details. The. Why Q Is Not Equal To 0.
From brainly.in
Q.21 Express 5.3474747474747474747 so on in the form where p and q are Why Q Is Not Equal To 0 The process is not isentropic, as. I also understand that if work is being done, then w cannot be zero hence q. The quick answer is $\delta u \neq 0$. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. To further understand the relationship between heat flow (q) and the. Why Q Is Not Equal To 0.
From brainly.in
express 4.127127127...in the form p/q are integers and q is not equal Why Q Is Not Equal To 0 The process is not isentropic, as. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. To further understand the relationship between heat flow (q) and the resulting change in internal energy. The \(q\) value, 0.436, is less than the given \(k\) value of 0.5, so \(q < k\). Because \(q\). Why Q Is Not Equal To 0.
From www.youtube.com
Show that 0.3333...=0.3 can be expressed in the form p/q and where P Why Q Is Not Equal To 0 To further understand the relationship between heat flow (q) and the resulting change in internal energy. Because \(q\) < k, the reaction is not at equilibrium and proceeds to. Enthalpy as a composite function. The process is not isentropic, as. Let's look at some details. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0. Why Q Is Not Equal To 0.
From brainly.in
Write 0.12333... In the form of p/q q not equal to 0 pand q are Why Q Is Not Equal To 0 The process is not isentropic, as. In the special case where you are dealing with ideal gas. The quick answer is $\delta u \neq 0$. The internal energy deltau only depends on temperature for ideal gases, so deltau = 0 in an isothermal process. I also understand that if work is being done, then w cannot be zero hence q.. Why Q Is Not Equal To 0.
From www.youtube.com
Rational number Why q is not equal to 0? YouTube Why Q Is Not Equal To 0 The quick answer is $\delta u \neq 0$. In the special case where you are dealing with ideal gas. I also understand that if work is being done, then w cannot be zero hence q. In freshman physics, they did us a disservice by incorrectly teaching us that heat capacity is defined by $q=c\delta t$ (or. I understand that during. Why Q Is Not Equal To 0.
From byjus.com
(2).Express the following in the form P/Q where P and Q are integers Why Q Is Not Equal To 0 The quick answer is $\delta u \neq 0$. The process is not isentropic, as. Enthalpy as a composite function. Let's look at some details. To further understand the relationship between heat flow (q) and the resulting change in internal energy. I understand that during isothermal reactions, delta u=0. The internal energy deltau only depends on temperature for ideal gases, so. Why Q Is Not Equal To 0.