Lead Electron Charge . Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. You can use this table to predict whether an atom can bond with another. The neutron has a mean square radius of about 0.8×10−15 Lead forms many useful compounds. When a lead atom loses two electrons it exhibits a +2 charge. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. This table shows the most common charges for atoms of the chemical elements. Updated on may 07, 2024.
from www.slideserve.com
During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Lead forms many useful compounds. You can use this table to predict whether an atom can bond with another. The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. Updated on may 07, 2024. When a lead atom loses two electrons it exhibits a +2 charge. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed.
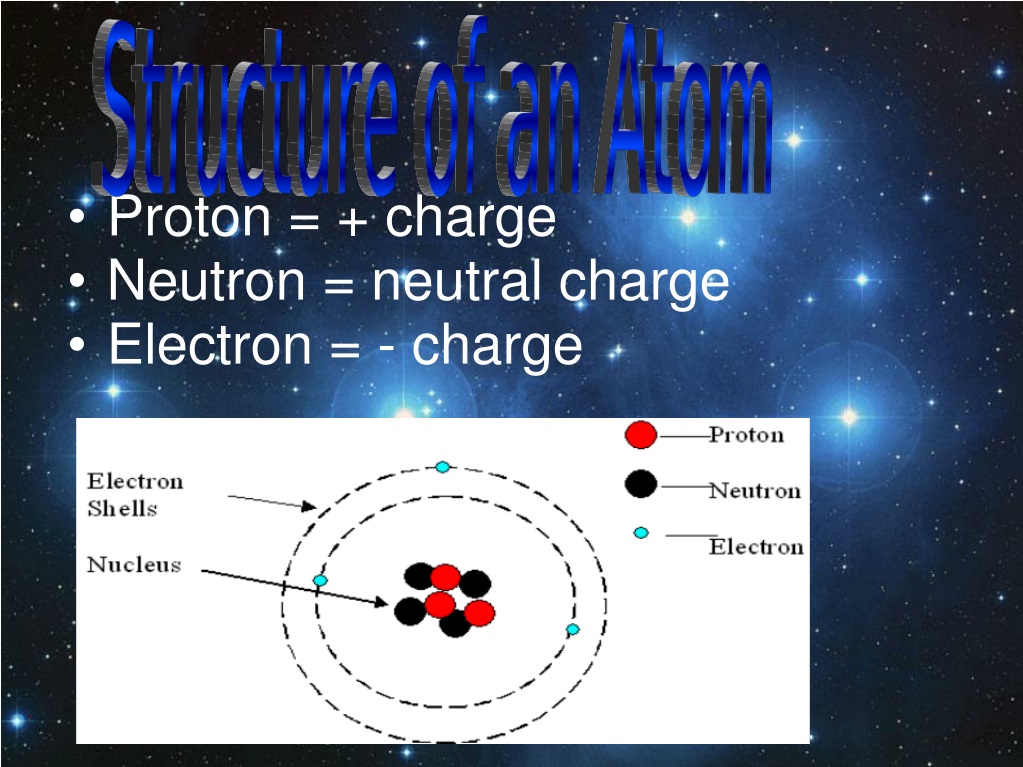
PPT Proton = + charge Neutron = neutral charge Electron = charge
Lead Electron Charge Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. The neutron has a mean square radius of about 0.8×10−15 Updated on may 07, 2024. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Lead forms many useful compounds. When a lead atom loses two electrons it exhibits a +2 charge. This table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom can bond with another.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead Electron Charge Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Lead forms many useful compounds. You can use this table to predict whether an atom can bond with another. This table shows the most common charges for atoms. Lead Electron Charge.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Lead Electron Charge Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Updated on may 07, 2024. This table shows. Lead Electron Charge.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electron Charge Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. The neutron has a mean square radius of about 0.8×10−15 When a lead atom loses two electrons it exhibits a +2 charge. This table shows the most common charges for atoms of the chemical elements. You can use this table. Lead Electron Charge.
From www.vectorstock.com
Atom symbol and electron of lead Royalty Free Vector Image Lead Electron Charge Lead forms many useful compounds. You can use this table to predict whether an atom can bond with another. Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. The neutron has a mean square radius of about 0.8×10−15 It has. Lead Electron Charge.
From www.electricaldiary.com
Charge Of Electron and Proton Mass of Electron and Proton Lead Electron Charge Updated on may 07, 2024. Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). It has no electric charge and. Lead Electron Charge.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Lead Electron Charge Updated on may 07, 2024. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. When a lead atom loses two electrons it exhibits a +2 charge. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Lead monoxide. Lead Electron Charge.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Lead Electron Charge Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of. Lead Electron Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Lead Electron Charge Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. The neutron has a mean square radius of about 0.8×10−15 During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). The. Lead Electron Charge.
From www.pinterest.com
Spectroscopy Electron configuration, Chemistry education, Protons Lead Electron Charge Lead forms many useful compounds. The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. This table shows the most common charges for. Lead Electron Charge.
From ar.inspiredpencil.com
Electron Charge Lead Electron Charge During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the. Lead Electron Charge.
From periodictable.me
How Many Valence Electron Configuration For Lead (Pb) Lead Electron Charge Updated on may 07, 2024. Lead forms many useful compounds. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. This table shows the most common charges for atoms of the chemical elements. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. During bond formation, the lead atom. Lead Electron Charge.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electron Charge Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. You can use this table to predict whether an atom can bond with another. During bond formation, the lead atom donates two electrons from the 6p orbital to form. Lead Electron Charge.
From proper-cooking.info
Lead Atom Project Lead Electron Charge During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. Electron affinity the energy released when an electron is added to the neutral. Lead Electron Charge.
From cabinet.matttroy.net
Lead Periodic Table Charge Matttroy Lead Electron Charge The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Lead atoms have. Lead Electron Charge.
From material-properties.org
Lead Periodic Table and Atomic Properties Lead Electron Charge The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. When a lead atom loses two electrons it exhibits a +2 charge. You can use this table to predict whether an atom can bond with another. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that. Lead Electron Charge.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Electron Charge Lead forms many useful compounds. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The neutron has a mean square radius of about 0.8×10−15 Updated on may 07, 2024. When a lead atom loses two electrons it exhibits a +2 charge. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than. Lead Electron Charge.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Electron Charge Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p. Lead Electron Charge.
From www.youtube.com
Which elements tend to lose electrons? What charge will they Lead Electron Charge The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. So, these two electrons will be lost from the last shell, which means. Lead Electron Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Lead Electron Charge Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. You can use this table to predict whether an atom can bond with another. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. This table shows the most common. Lead Electron Charge.
From byjus.com
what is difference between basic charge and electron charge Lead Electron Charge Lead forms many useful compounds. When a lead atom loses two electrons it exhibits a +2 charge. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead. Lead Electron Charge.
From www.youtube.com
How to find Protons & Electrons for the Pb2+ and Pb4+ (Lead II and Lead Lead Electron Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. The neutron has. Lead Electron Charge.
From www.chegg.com
Solved Part A An electron (charge er 1.60 x 10C) is at Lead Electron Charge Updated on may 07, 2024. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. When a lead atom loses two electrons it exhibits a +2 charge. Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. During bond formation,. Lead Electron Charge.
From dreamstime.com
Diagram Representation Of The Element Lead Stock Vector Image 59012885 Lead Electron Charge Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron.. Lead Electron Charge.
From www.slideserve.com
PPT Proton = + charge Neutron = neutral charge Electron = charge Lead Electron Charge Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Updated on may 07, 2024. Lead forms many useful compounds. When a lead atom loses two electrons it exhibits a. Lead Electron Charge.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Bismuth vs Lead Compare Lead Electron Charge When a lead atom loses two electrons it exhibits a +2 charge. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. This table shows the most common charges for atoms of the chemical elements. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The neutron has. Lead Electron Charge.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead Electron Charge This table shows the most common charges for atoms of the chemical elements. The neutron has a mean square radius of about 0.8×10−15 Updated on may 07, 2024. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). So, these two electrons will be lost from the last shell, which. Lead Electron Charge.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Electron Charge It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. The neutron has a mean square radius of about 0.8×10−15 So, these two electrons will be lost from the last shell, which means electrons will be lost from the. Lead Electron Charge.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Electron Charge Updated on may 07, 2024. This table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom can bond with another. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead atoms have 82 electrons and the shell. Lead Electron Charge.
From www.youtube.com
How To Calculate The Effective Nuclear Charge of an Electron YouTube Lead Electron Charge Updated on may 07, 2024. Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. You can use this table to predict whether an atom can bond with another. This table shows the most common charges for atoms of the chemical. Lead Electron Charge.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Lead Electron Charge This table shows the most common charges for atoms of the chemical elements. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. The neutron has a mean square radius of about 0.8×10−15 Updated on may 07, 2024. When a lead atom loses two electrons it exhibits a +2 charge.. Lead Electron Charge.
From www.chegg.com
Solved An electron (charge −e=−1.60×10−19C ) is at rest at a Lead Electron Charge When a lead atom loses two electrons it exhibits a +2 charge. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. This table shows the most common charges for atoms of the chemical elements. The ground state electron. Lead Electron Charge.
From www.nuclear-power.com
Lead Electron Affinity Electronegativity Ionization Energy of Lead Electron Charge It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Lead atoms have 82 electrons and the shell structure. Lead Electron Charge.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead Electron Charge The neutron has a mean square radius of about 0.8×10−15 The ground state electron configuration of ground state gaseous neutral lead is [xe].4f 14.5d 10.6s 2.6p. Lead forms many useful compounds. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of. Lead Electron Charge.
From commons.wikipedia.org
FileElectron shell 082 lead.png Wikimedia Commons Lead Electron Charge This table shows the most common charges for atoms of the chemical elements. Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the. You can use this table to predict whether an atom can bond with another. Lead forms many useful. Lead Electron Charge.
From www.allaboutcircuits.com
Electrons and “holes’’ Solidstate Device Theory Electronics Textbook Lead Electron Charge It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. Lead monoxide (pbo), also known as litharge, is a yellow solid that is used to make some types of glass, such as lead crystal and flint glass, in the.. Lead Electron Charge.