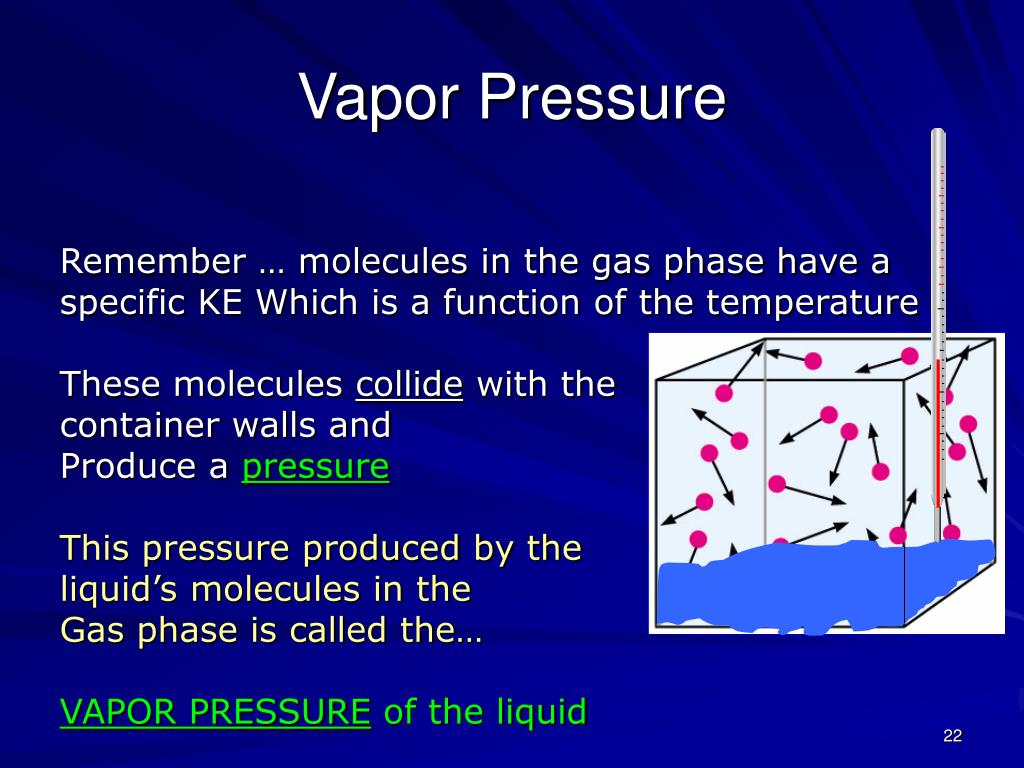
What Is Vapor Pressure Chemistry . When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure is a property of a liquid based on. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);
from www.slideserve.com
Vapor pressure is a property of a liquid based on. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with.
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574
What Is Vapor Pressure Chemistry Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is a property of a liquid based on. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with.
From www.youtube.com
Raoults Law and Vapor Pressure Chemistry Tutorial YouTube What Is Vapor Pressure Chemistry The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in. What Is Vapor Pressure Chemistry.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapor Pressure Chemistry The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour. What Is Vapor Pressure Chemistry.
From www.youtube.com
CHEMISTRY 201 Using the ClausiusClapeyron equation to solve for vapor What Is Vapor Pressure Chemistry Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. The vapor pressure is a measure. What Is Vapor Pressure Chemistry.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Is Vapor Pressure Chemistry When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. That is, the pressure of the vapor resulting from evaporation. What Is Vapor Pressure Chemistry.
From www.ck12.org
Vapor Pressure Overview ( Video ) Chemistry CK12 Foundation What Is Vapor Pressure Chemistry That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its. What Is Vapor Pressure Chemistry.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapor Pressure Chemistry Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at Vapor pressure is a property of. What Is Vapor Pressure Chemistry.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID2755893 What Is Vapor Pressure Chemistry The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in. What Is Vapor Pressure Chemistry.
From saylordotorg.github.io
Vapor Pressure What Is Vapor Pressure Chemistry Vapor pressure is a property of a liquid based on. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. What Is Vapor Pressure Chemistry.
From www.slideserve.com
PPT Syllabus Chemistry 102 Spring 2011 Sec. 501, 503 (MWF 8850, 12 What Is Vapor Pressure Chemistry Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure or equilibrium vapor pressure. What Is Vapor Pressure Chemistry.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts What Is Vapor Pressure Chemistry The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure is a property of a liquid based on. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. That is, the pressure of the vapor resulting. What Is Vapor Pressure Chemistry.
From quizizz.com
Vapor Pressure Practice (Gases pg 22) questions & answers for quizzes What Is Vapor Pressure Chemistry That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. Vapor pressure is a property of a liquid based on. The vapor pressure is a. What Is Vapor Pressure Chemistry.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Vapor Pressure Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is a measure of the tendency of a material to change into the gaseous or. What Is Vapor Pressure Chemistry.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Chemistry That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. Vapor pressure is a property of a liquid based on. Vapour pressure, also known as. What Is Vapor Pressure Chemistry.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Chemistry That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a property of a liquid based on. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure or equilibrium vapor pressure is the pressure. What Is Vapor Pressure Chemistry.
From sciencenotes.org
Clausius Clapeyron Equation What Is Vapor Pressure Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. That is, the pressure of the vapor resulting from evaporation of a. What Is Vapor Pressure Chemistry.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Is Vapor Pressure Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure or equilibrium vapor pressure is the pressure of a. What Is Vapor Pressure Chemistry.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co What Is Vapor Pressure Chemistry Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure is a property of a liquid based on. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. The vapor. What Is Vapor Pressure Chemistry.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Is Vapor Pressure Chemistry Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. The vapor pressure is a measure. What Is Vapor Pressure Chemistry.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Is Vapor Pressure Chemistry Vapor pressure is a property of a liquid based on. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is a measure of the tendency of a material to change into. What Is Vapor Pressure Chemistry.
From www.dreamstime.com
Vapor Pressure with Molecule Movement in Closed Container Outline What Is Vapor Pressure Chemistry Vapor pressure is a property of a liquid based on. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its. What Is Vapor Pressure Chemistry.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson What Is Vapor Pressure Chemistry Vapor pressure is a property of a liquid based on. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure. What Is Vapor Pressure Chemistry.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 What Is Vapor Pressure Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its. What Is Vapor Pressure Chemistry.
From www.youtube.com
Vapour Pressure & Raoults Law Chemistry Class 12 IIT JEE Main What Is Vapor Pressure Chemistry Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system. What Is Vapor Pressure Chemistry.
From guides.hostos.cuny.edu
Chapter 3 Solids and Liquids CHE 110 Introduction to Chemistry What Is Vapor Pressure Chemistry When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a. What Is Vapor Pressure Chemistry.
From ar.inspiredpencil.com
Vapor Pressure Example What Is Vapor Pressure Chemistry Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at By. What Is Vapor Pressure Chemistry.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Chemistry Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid). What Is Vapor Pressure Chemistry.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Chemistry Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at Vapor pressure is a property of a liquid based on. The vapor pressure is a measure of the pressure (force per unit area) exerted. What Is Vapor Pressure Chemistry.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure Chemistry When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); By doing so, they generate a population of molecules in the vapor phase above the. What Is Vapor Pressure Chemistry.
From socratic.org
What is the relation between critical temperature and boiling point or What Is Vapor Pressure Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a. What Is Vapor Pressure Chemistry.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure Chemistry Vapor pressure is a property of a liquid based on. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure, also known as vapour equilibrium pressure, can. What Is Vapor Pressure Chemistry.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure Chemistry That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a. What Is Vapor Pressure Chemistry.
From www.youtube.com
Colligative properties Vapor pressure lowering YouTube What Is Vapor Pressure Chemistry The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces. What Is Vapor Pressure Chemistry.
From ar.inspiredpencil.com
Vapor Pressure Example What Is Vapor Pressure Chemistry Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure is a measure of. What Is Vapor Pressure Chemistry.
From scienceinfo.com
Lowering of Vapor Pressure A Colligative property What Is Vapor Pressure Chemistry The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Vapor pressure is a property of a liquid based on. Vapor pressure or equilibrium vapor pressure is. What Is Vapor Pressure Chemistry.
From amariqoleon.blogspot.com
What is Vapor Pressure AmariqoLeon What Is Vapor Pressure Chemistry Vapor pressure is a property of a liquid based on. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at That is, the pressure of the vapor resulting from evaporation of a liquid (or. What Is Vapor Pressure Chemistry.