Titration To Determine The Concentration Of A Base . Ma is the molarity of the acid, while mb is the molarity of the base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. M av a = m bv b. Va and vb are the volumes of the acid and base, respectively. When the reaction between the. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. It is based on the neutralization reaction, where.
from slidetodoc.com
Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. When the reaction between the. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Va and vb are the volumes of the acid and base, respectively. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is based on the neutralization reaction, where. Ma is the molarity of the acid, while mb is the molarity of the base. M av a = m bv b. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or.
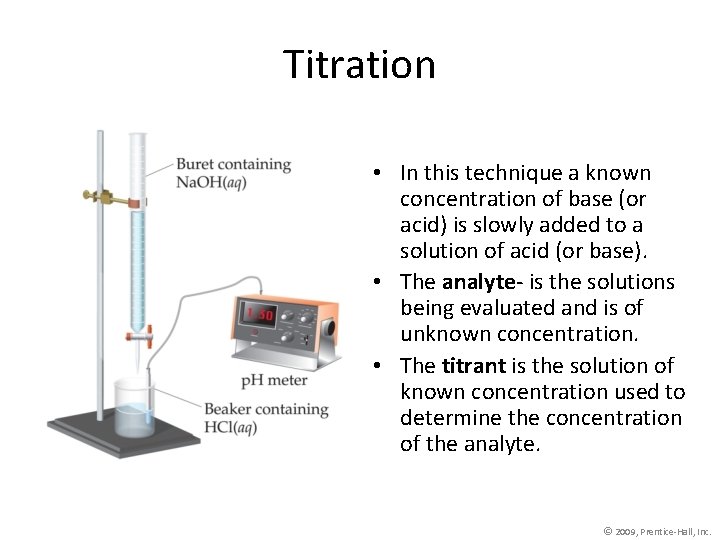
Acid Base Titration Titration In this technique a
Titration To Determine The Concentration Of A Base As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. M av a = m bv b. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Va and vb are the volumes of the acid and base, respectively. Ma is the molarity of the acid, while mb is the molarity of the base. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. It is based on the neutralization reaction, where. When the reaction between the.
From www.studocu.com
Acid Bases HL Paper 2 A student performs a titration to determine the concentration of Titration To Determine The Concentration Of A Base Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Va and. Titration To Determine The Concentration Of A Base.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Khan Academy YouTube Titration To Determine The Concentration Of A Base It is based on the neutralization reaction, where. Va and vb are the volumes of the acid and base, respectively. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions. Titration To Determine The Concentration Of A Base.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration To Determine The Concentration Of A Base A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or.. Titration To Determine The Concentration Of A Base.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 Titration To Determine The Concentration Of A Base M av a = m bv b. It is based on the neutralization reaction, where. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. As seen in the chapter on the stoichiometry. Titration To Determine The Concentration Of A Base.
From studylib.net
AcidBase Titration Curve Titration To Determine The Concentration Of A Base Va and vb are the volumes of the acid and base, respectively. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. When the reaction between. Titration To Determine The Concentration Of A Base.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d) TRIPLE Teaching Resources Titration To Determine The Concentration Of A Base It is based on the neutralization reaction, where. Va and vb are the volumes of the acid and base, respectively. M av a = m bv b. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Ma is the molarity of the acid, while mb is the molarity of. Titration To Determine The Concentration Of A Base.
From theedge.com.hk
Chemistry How To Titration The Edge Titration To Determine The Concentration Of A Base Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Ma is the molarity of the acid, while mb is the molarity of the base. Va and vb are the volumes of the acid and base, respectively. When the reaction between the. It is based on the neutralization reaction, where. Titration is a. Titration To Determine The Concentration Of A Base.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration To Determine The Concentration Of A Base When the reaction between the. It is based on the neutralization reaction, where. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. M av a = m bv b.. Titration To Determine The Concentration Of A Base.
From slidetodoc.com
Titrations Titration A known concentration of base or Titration To Determine The Concentration Of A Base A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Va and vb are the volumes of the acid and base, respectively. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Ma is the molarity of the acid, while mb is the. Titration To Determine The Concentration Of A Base.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration To Determine The Concentration Of A Base As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base.. Titration To Determine The Concentration Of A Base.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Titration To Determine The Concentration Of A Base Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. When the reaction between the. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively. Titration To Determine The Concentration Of A Base.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution YouTube Titration To Determine The Concentration Of A Base Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Va and vb are the volumes of the acid and base, respectively. Ma is the molarity of the acid, while mb is the molarity of the base. As seen in the chapter on the stoichiometry of chemical reactions, titrations. Titration To Determine The Concentration Of A Base.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration To Determine The Concentration Of A Base A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. M av a = m bv b. It is based on the neutralization reaction, where. When the reaction between the. Ma is the molarity of the acid, while mb is the molarity of the base. As seen in the chapter. Titration To Determine The Concentration Of A Base.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration To Determine The Concentration Of A Base Ma is the molarity of the acid, while mb is the molarity of the base. It is based on the neutralization reaction, where. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Va and vb are the volumes of the acid and base, respectively. A titration is a laboratory technique used to. Titration To Determine The Concentration Of A Base.
From www.sliderbase.com
Titration Titration To Determine The Concentration Of A Base A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. When the reaction between the. It is based on the neutralization reaction, where. Titration is a method. Titration To Determine The Concentration Of A Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration To Determine The Concentration Of A Base A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Ma is the molarity of the acid, while mb is the molarity of the base. Va and vb are the volumes of the acid and base, respectively. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl). Titration To Determine The Concentration Of A Base.
From mungfali.com
Acid Base Titration Calculation Titration To Determine The Concentration Of A Base Va and vb are the volumes of the acid and base, respectively. It is based on the neutralization reaction, where. Ma is the molarity of the acid, while mb is the molarity of the base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating. Titration To Determine The Concentration Of A Base.
From www.youtube.com
Finding concentration of acid or base given amount needed to titrate and molarity YouTube Titration To Determine The Concentration Of A Base A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is based on the neutralization reaction, where. M av a = m bv b. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. As seen in the chapter on the stoichiometry. Titration To Determine The Concentration Of A Base.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration To Determine The Concentration Of A Base Va and vb are the volumes of the acid and base, respectively. M av a = m bv b. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. As seen in. Titration To Determine The Concentration Of A Base.
From www.numerade.com
SOLVED REPORT SHEET AcidBase Titration LAB 12 Concentration of Acetic Acid in Vinegar Brand Titration To Determine The Concentration Of A Base Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. It is based on the neutralization reaction, where. When the reaction between the. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Va and vb are the volumes of. Titration To Determine The Concentration Of A Base.
From www.science-revision.co.uk
Titrations Titration To Determine The Concentration Of A Base Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. When the reaction between the. Va and vb are the volumes of the acid and base, respectively. M av a = m. Titration To Determine The Concentration Of A Base.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration To Determine The Concentration Of A Base When the reaction between the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Va and vb are the volumes of the acid and base, respectively. M av a = m bv. Titration To Determine The Concentration Of A Base.
From www.slideserve.com
PPT AcidBase Titrations A. Definition volumetric analysis of the concentration of an unknown Titration To Determine The Concentration Of A Base It is based on the neutralization reaction, where. Va and vb are the volumes of the acid and base, respectively. When the reaction between the. M av a = m bv b. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. A titration is a laboratory technique used. Titration To Determine The Concentration Of A Base.
From mammothmemory.net
Titration is an experiment to determine concentrations Titration To Determine The Concentration Of A Base Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Ma is the molarity of the acid, while mb is the molarity of the base. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. It is based on the neutralization reaction,. Titration To Determine The Concentration Of A Base.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation ID4401864 Titration To Determine The Concentration Of A Base Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. M av a = m bv b. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Ma is the molarity of the acid, while mb is the molarity of the base. Va. Titration To Determine The Concentration Of A Base.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Foundation Titration To Determine The Concentration Of A Base M av a = m bv b. Ma is the molarity of the acid, while mb is the molarity of the base. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. It is based on the neutralization reaction, where. A titration is a laboratory technique used. Titration To Determine The Concentration Of A Base.
From www.nagwa.com
Question Video Determining the Percentage of Base in a Mixture by Titration Nagwa Titration To Determine The Concentration Of A Base Va and vb are the volumes of the acid and base, respectively. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. When the reaction between the. Ma is the molarity of the acid, while mb is the molarity of the base. It is based on the neutralization reaction, where. A titration is. Titration To Determine The Concentration Of A Base.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Presentation ID1995345 Titration To Determine The Concentration Of A Base Va and vb are the volumes of the acid and base, respectively. M av a = m bv b. It is based on the neutralization reaction, where. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl). Titration To Determine The Concentration Of A Base.
From www.savemyexams.com
AcidAlkali Titrations (2.6.3) Edexcel IGCSE Chemistry Revision Notes 2019 Save My Exams Titration To Determine The Concentration Of A Base M av a = m bv b. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Ma is the molarity of the acid, while mb is the molarity of the base. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze. Titration To Determine The Concentration Of A Base.
From slidetodoc.com
Acid Base Titration Titration In this technique a Titration To Determine The Concentration Of A Base M av a = m bv b. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. When the reaction between the. It is based on the neutralization reaction, where. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Ma is. Titration To Determine The Concentration Of A Base.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration To Determine The Concentration Of A Base M av a = m bv b. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Va and vb are the volumes of the acid and base, respectively. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is based on. Titration To Determine The Concentration Of A Base.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry Titration To Determine The Concentration Of A Base A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. M av a = m bv b. It is based on the neutralization reaction, where. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Ma is the molarity. Titration To Determine The Concentration Of A Base.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration To Determine The Concentration Of A Base It is based on the neutralization reaction, where. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration is a laboratory technique used to. Titration To Determine The Concentration Of A Base.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration To Determine The Concentration Of A Base Va and vb are the volumes of the acid and base, respectively. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. When the reaction between the. M av a = m bv b. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. Titration To Determine The Concentration Of A Base.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration To Determine The Concentration Of A Base Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Va and vb are the volumes of the acid and base, respectively. Ma is the molarity of the acid, while mb is the molarity of the base. It is based on the neutralization reaction, where. As seen in the. Titration To Determine The Concentration Of A Base.