Chlorinated Alkane . Also we disucss free radicals and their stability in. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. When alkanes larger than ethane are halogenated, isomeric products are formed. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by.
from www.numerade.com
For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. When alkanes larger than ethane are halogenated, isomeric products are formed. Also we disucss free radicals and their stability in.
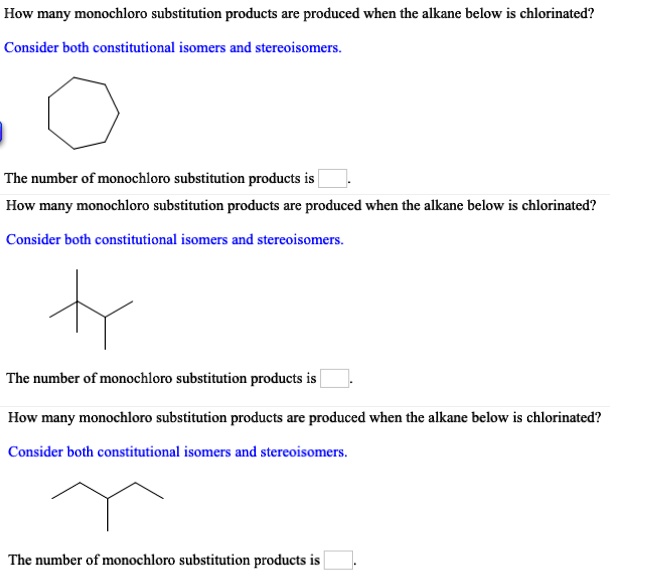
SOLVED How many monochloro substitution products are produced when the
Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. When alkanes larger than ethane are halogenated, isomeric products are formed. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Also we disucss free radicals and their stability in. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in.
From www.chegg.com
Solved How many monochloro substitution products are Chlorinated Alkane Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products. Chlorinated Alkane.
From www.arnoldproducts.co.nz
20KG CIP POWDER H/D CHLORINATED ALKALINE DETERGENT Arnold Products Chlorinated Alkane For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. Also we disucss free radicals and their stability in. In this tutorial, we consider methane. Chlorinated Alkane.
From www.chegg.com
Solved How many monochloro substitution products are Chlorinated Alkane When alkanes larger than ethane are halogenated, isomeric products are formed. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. For an alkane with only one. Chlorinated Alkane.
From www.nvchemicals.com.au
Chlorinated Detergent chlorinated alkaline detergent Chlorinated Alkane In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. When alkanes larger than ethane are halogenated, isomeric products are formed. Depending on the time allowed and the relative amounts. Chlorinated Alkane.
From www.scribd.com
Status of Medium and LongChain Chlorinated Paraffins PDF Alkane Chlorinated Alkane In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Also we disucss free radicals and their stability in. Depending on the time allowed and the. Chlorinated Alkane.
From buckcreekhops.com
Brewfoam, Foaming Chlorinated Alkaline Cleaner Buck Creek Distributing Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. When alkanes larger than ethane are halogenated, isomeric products are formed. Unlike the complex transformations of combustion,. Chlorinated Alkane.
From www.ecochem.co.nz
Force Chlorinated Alkaline Foam Cleaner Ecochem Limited Chlorinated Alkane Also we disucss free radicals and their stability in. When alkanes larger than ethane are halogenated, isomeric products are formed. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction. Chlorinated Alkane.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a. Chlorinated Alkane.
From proecoquimicas.com
Foaming alkaline chlorinated cleaner to sanitize surfaces Chlorinated Alkane Also we disucss free radicals and their stability in. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. When alkanes larger than ethane are halogenated, isomeric products are formed. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in.. Chlorinated Alkane.
From www.chegg.com
Solved How many monochloro substitution products are Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. When alkanes larger than ethane are halogenated, isomeric products are formed. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Also we disucss free radicals and their stability. Chlorinated Alkane.
From www.chegg.com
Solved How many monochloro substitution products are Chlorinated Alkane In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution. Chlorinated Alkane.
From easy-schule.de
Alkane Definition & Zusammenfassung Easy Schule Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. When alkanes larger than ethane are halogenated, isomeric products are formed. For an alkane with only one. Chlorinated Alkane.
From procure-net.com
Premium Quality 2Chlorobutane PharmaceuticalGrade Chlorinated Chlorinated Alkane Also we disucss free radicals and their stability in. When alkanes larger than ethane are halogenated, isomeric products are formed. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product. Chlorinated Alkane.
From averex.com.my
Chlorinated Alkaline Foam Cleaner (MAXICLEAN FC6) AVEREX Cleaning Chlorinated Alkane When alkanes larger than ethane are halogenated, isomeric products are formed. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Also we disucss free radicals and their stability in. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution. Chlorinated Alkane.
From byjus.com
34. Which of the following alkane upon Di chlorination can give only Chlorinated Alkane In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Also we disucss free radicals and their stability in. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Unlike the complex transformations of combustion, the. Chlorinated Alkane.
From chenxuchem2001.en.made-in-china.com
Solid Chlorinated Alkane 70 Powder China Solid Chlorinated Alkane Chlorinated Alkane When alkanes larger than ethane are halogenated, isomeric products are formed. Also we disucss free radicals and their stability in. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since. Chlorinated Alkane.
From www.hophavoc.com
Brewfoam Foaming Chlorinated Alkaline Cleaner Havoc Brew Supply Chlorinated Alkane For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Also we disucss free radicals and their stability in. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. When alkanes larger than ethane are. Chlorinated Alkane.
From www.daviesway.com.au
ChlorKlenz Heavy Duty Chlorinated Alkaline Dairy Detergent Dasco Chlorinated Alkane When alkanes larger than ethane are halogenated, isomeric products are formed. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. Also we disucss free radicals and their stability in. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since. Chlorinated Alkane.
From druginfohq.com
CHLORINATED ALKALINE DETERGENT GALLON X 3785 LITERS Chlorinated Alkane In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Also we disucss free radicals and their stability in. When alkanes larger than ethane are halogenated, isomeric products are formed. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane. Chlorinated Alkane.
From averex.com.my
Chlorinated Alkaline Cleaner (MAXICLEAN CP10) AVEREX Cleaning Chlorinated Alkane Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen. Chlorinated Alkane.
From www.youtube.com
Physical & Chemical Properties of Alkanes 3 Mechanism of chlorination Chlorinated Alkane Also we disucss free radicals and their stability in. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. When alkanes larger than ethane are. Chlorinated Alkane.
From www.youtube.com
How many mono chlorinated products mayt be obtained wthen the alkane Chlorinated Alkane Also we disucss free radicals and their stability in. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. For an alkane with only one type of hydrogen, the. Chlorinated Alkane.
From www.chegg.com
Solved How many monochloro substitution products are Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. Also we disucss free radicals and their stability in. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. For an alkane with only one type of. Chlorinated Alkane.
From chenxuchem2001.en.made-in-china.com
Solid Chlorinated Alkane Chlorinated Paraffin 70 for Mineral Filled Chlorinated Alkane In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen. Chlorinated Alkane.
From procure-net.com
1Chloroctadecane HighQuality Chlorinated Alkane for Various Chlorinated Alkane Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Also we disucss free radicals and their stability in. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Depending on the time allowed and the relative amounts of the. Chlorinated Alkane.
From procure-net.com
Tripherylchloromethane Premium PharmaceuticalGrade Chlorinated Chlorinated Alkane In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen. Chlorinated Alkane.
From www.numerade.com
SOLVED How many monochloro substitution products are produced when the Chlorinated Alkane Also we disucss free radicals and their stability in. When alkanes larger than ethane are halogenated, isomeric products are formed. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms. Chlorinated Alkane.
From procure-net.com
1Chloroctadecane HighQuality Chlorinated Alkane for Various Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. Also we disucss free radicals and their stability in. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Unlike the complex transformations of combustion,. Chlorinated Alkane.
From procure-net.com
1Chlorobutane High Purity, Pharmaceutical Grade Chlorinated Alkane Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. When alkanes larger than ethane are halogenated, isomeric products are formed. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Also we disucss free radicals and their stability. Chlorinated Alkane.
From hambydairysupply.com
Solution® Chlorinated Alkaline Liquid CIP Detergent 5 gallon Hamby Chlorinated Alkane For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. Also we disucss free radicals and their stability in. In this tutorial, we consider methane. Chlorinated Alkane.
From www.amazon.com
Ecolab Oasis Enforce SelfFoaming Chlorinated Alkaline Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. Also we disucss free radicals and their stability in. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. For an alkane with only one type of hydrogen, the. Chlorinated Alkane.
From www.foodsafetychemicals.com
Foam 364 Liquid Alkaline Chlorinated Foam Cleaner Food Safety Chemicals Chlorinated Alkane For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. Also we disucss free radicals and their stability in. When alkanes larger than ethane are halogenated, isomeric products are formed. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution. Chlorinated Alkane.
From www.onlinemathlearning.com
Organic Chemistry IGCSE Chemistry (solutions, examples, worksheets Chlorinated Alkane Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. In this tutorial, we consider methane as the alkane compound and study about it's chlorination reactions steps, products and the mechanism. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution. Chlorinated Alkane.
From chenxuchem2001.en.made-in-china.com
Industrial Grade Chlorinated Alkane Powder Chlorinated Paraffin and Chlorinated Alkane When alkanes larger than ethane are halogenated, isomeric products are formed. Also we disucss free radicals and their stability in. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product. Chlorinated Alkane.
From diversey.co.uk
DivoCIP VC94 200L Soft water, low foam chlorinated alkaline detergent Chlorinated Alkane When alkanes larger than ethane are halogenated, isomeric products are formed. Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by. Also we disucss free radicals and their stability in. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction. Chlorinated Alkane.