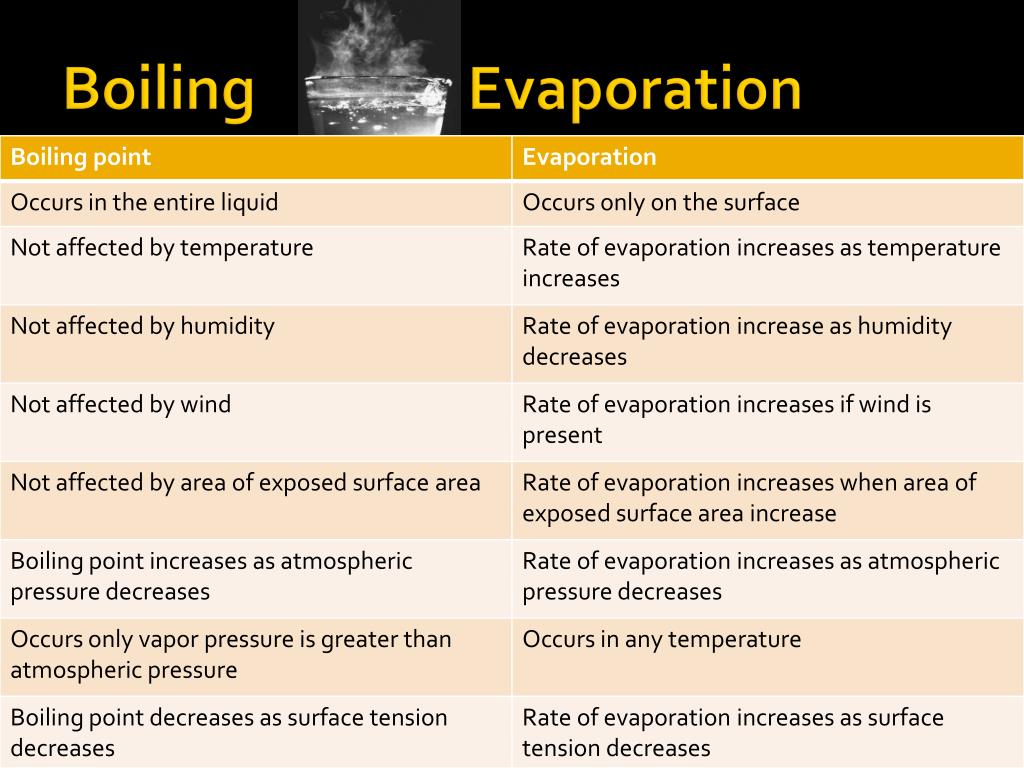
Water Not Boiling Just Evaporation . during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. Rather, it is the temperature at which the saturation vapor. how does water evaporate without boiling? in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. when liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. why does water boil at \(100^oc\)? if the water was warmer, it would have evaporated faster. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state.
from www.slideserve.com
Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. when liquid water meets dry air, it is not in equilibrium; in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. if the water was warmer, it would have evaporated faster. why does water boil at \(100^oc\)? Rather, it is the temperature at which the saturation vapor.
PPT Evaporation and Boiling Point PowerPoint Presentation, free
Water Not Boiling Just Evaporation why does water boil at \(100^oc\)? during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. when liquid water meets dry air, it is not in equilibrium; why does water boil at \(100^oc\)? You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. how does water evaporate without boiling? if air pressure is high on the surface of a body of water, then the water will not evaporate easily. if the water was warmer, it would have evaporated faster. Rather, it is the temperature at which the saturation vapor. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state.
From www.bbc.co.uk
Evaporation BBC Bitesize Water Not Boiling Just Evaporation if the water was warmer, it would have evaporated faster. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. when liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough. Water Not Boiling Just Evaporation.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID9099661 Water Not Boiling Just Evaporation In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. how does water evaporate without boiling? if the water was warmer, it would have evaporated faster. when liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in. Water Not Boiling Just Evaporation.
From sites.google.com
Y7 Science helper Reference page Water Not Boiling Just Evaporation how does water evaporate without boiling? In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. why does water boil at \(100^oc\)? during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. if air pressure. Water Not Boiling Just Evaporation.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free Water Not Boiling Just Evaporation in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. Rather, it is the temperature at which the saturation vapor. Water molecules evaporate off the surface until the amount of water. Water Not Boiling Just Evaporation.
From www.aplustopper.com
How can we Separate a Mixture of a Solid and a Liquid using Evaporation Water Not Boiling Just Evaporation how does water evaporate without boiling? the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. why does water boil at \(100^oc\)? during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. in an. Water Not Boiling Just Evaporation.
From www.slideserve.com
PPT Liquids & Vapor Pressure PowerPoint Presentation ID4196264 Water Not Boiling Just Evaporation why does water boil at \(100^oc\)? how does water evaporate without boiling? when liquid water meets dry air, it is not in equilibrium; In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. Rather, it is the temperature at which the saturation vapor. if air pressure is high. Water Not Boiling Just Evaporation.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Water Not Boiling Just Evaporation how does water evaporate without boiling? why does water boil at \(100^oc\)? Rather, it is the temperature at which the saturation vapor. In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure. Water Not Boiling Just Evaporation.
From www.merchantnavydecoded.com
What is Evaporation and boiling? Merchant Navy Decoded Water Not Boiling Just Evaporation In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous. Water Not Boiling Just Evaporation.
From pediaa.com
Difference Between Evaporation and Boiling Water Not Boiling Just Evaporation when liquid water meets dry air, it is not in equilibrium; in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. how does water evaporate without boiling? . Water Not Boiling Just Evaporation.
From www.nsta.org
Q What’s the difference between evaporation and boiling? NSTA Water Not Boiling Just Evaporation in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. how does water evaporate without boiling? when liquid water meets dry air, it is not in. Water Not Boiling Just Evaporation.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Water Not Boiling Just Evaporation when liquid water meets dry air, it is not in equilibrium; if the water was warmer, it would have evaporated faster. during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. if air pressure is high on the surface of a body of. Water Not Boiling Just Evaporation.
From themasterchemistry.com
Difference Between Evaporation And BoilingEvaporation Vs Boiling Water Not Boiling Just Evaporation Rather, it is the temperature at which the saturation vapor. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. In contrast, boiling occurs only. Water Not Boiling Just Evaporation.
From www.differencebetween.com
Difference Between Boiling Point and Evaporation Compare the Water Not Boiling Just Evaporation Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. if the water was warmer, it would have evaporated faster. how does water evaporate without boiling? You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm.. Water Not Boiling Just Evaporation.
From www.robins.af.mil
UPDATE Boil Water Advisory > Robins Air Force Base > Article Display Water Not Boiling Just Evaporation during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. how does water evaporate without boiling? You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. Water molecules evaporate off the surface until. Water Not Boiling Just Evaporation.
From 88guru.com
Difference Between Evaporation and Boiling Water Not Boiling Just Evaporation In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. why does water boil at \(100^oc\)? the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times. Water Not Boiling Just Evaporation.
From stock.adobe.com
evaporation and boiling point of water . vector vector de Stock Adobe Water Not Boiling Just Evaporation when liquid water meets dry air, it is not in equilibrium; In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. how does water evaporate without. Water Not Boiling Just Evaporation.
From www.slideserve.com
PPT Chapter 11. States of Matter PowerPoint Presentation, free Water Not Boiling Just Evaporation if the water was warmer, it would have evaporated faster. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. in an open container in an atmosphere. Water Not Boiling Just Evaporation.
From www.nsta.org
Q What’s the difference between evaporation and boiling? NSTA Water Not Boiling Just Evaporation if air pressure is high on the surface of a body of water, then the water will not evaporate easily. why does water boil at \(100^oc\)? You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. how does water evaporate without boiling? Rather, it is. Water Not Boiling Just Evaporation.
From www.youtube.com
Boiling and Evaporation YouTube Water Not Boiling Just Evaporation Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. if the water was warmer, it would have evaporated faster. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. You will note from table that the vapor pressure of. Water Not Boiling Just Evaporation.
From missvickie.com
6 Ways To Fix Water Not Boiling Miss Vickie Water Not Boiling Just Evaporation in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. the boiling temperature of a liquid is not the temperature at which it can. Water Not Boiling Just Evaporation.
From www.scienceabc.com
Boiling Water Science Why Does Water Make Noise Before It Boils? Water Not Boiling Just Evaporation during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. Rather, it is the temperature at which the saturation vapor. if the water was warmer, it. Water Not Boiling Just Evaporation.
From www.youtube.com
Difference Between Evaporation and Boiling MDCat and JEE main Water Not Boiling Just Evaporation Rather, it is the temperature at which the saturation vapor. during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. In contrast, boiling occurs. Water Not Boiling Just Evaporation.
From schoolings.org
Evaporation And Boiling Meaning, Differences, Factors Affecting Water Not Boiling Just Evaporation In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. when liquid water meets dry air, it is not in equilibrium; why does water boil at \(100^oc\)? Rather, it is the temperature at which the saturation vapor. if air pressure is high on the surface of a body of. Water Not Boiling Just Evaporation.
From www.toppr.com
Differences Between Evaporation and Boiling in Chemistry Water Not Boiling Just Evaporation In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. if the water was warmer, it would have evaporated faster. how does water evaporate without boiling? why does water boil. Water Not Boiling Just Evaporation.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free Water Not Boiling Just Evaporation when liquid water meets dry air, it is not in equilibrium; the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. if air pressure is high on the. Water Not Boiling Just Evaporation.
From askanydifference.com
Boiling vs Evaporation Difference and Comparison Water Not Boiling Just Evaporation why does water boil at \(100^oc\)? the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together. if air pressure is high on the surface of. Water Not Boiling Just Evaporation.
From missvickie.com
6 Ways To Fix Water Not Boiling Miss Vickie Water Not Boiling Just Evaporation when liquid water meets dry air, it is not in equilibrium; You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. how does water evaporate without boiling? . Water Not Boiling Just Evaporation.
From thewaterfiltermarket.com
How Long Does It Take for Water To Evaporate? Water Filter Market Water Not Boiling Just Evaporation You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. if the water was warmer, it would have evaporated faster. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. Rather, it is the temperature at. Water Not Boiling Just Evaporation.
From www.wisegeek.com
What is Evaporation? (with pictures) Water Not Boiling Just Evaporation when liquid water meets dry air, it is not in equilibrium; in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. if the water was warmer,. Water Not Boiling Just Evaporation.
From circuitdiagramlows.z22.web.core.windows.net
Phase Change Diagram For Water Water Not Boiling Just Evaporation the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. during boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link. Water Not Boiling Just Evaporation.
From www.diffzy.com
Evaporation vs Boiling What's the Difference (With Table) Water Not Boiling Just Evaporation why does water boil at \(100^oc\)? when liquid water meets dry air, it is not in equilibrium; how does water evaporate without boiling? You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. Water molecules evaporate off the surface until the amount of water in. Water Not Boiling Just Evaporation.
From dxowlbddi.blob.core.windows.net
Explain The Evaporation Process at James Ligon blog Water Not Boiling Just Evaporation In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. if the water was warmer, it would have evaporated faster. You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. Water molecules evaporate off the surface until the amount. Water Not Boiling Just Evaporation.
From missvickie.com
6 Ways To Fix Water Not Boiling Miss Vickie Water Not Boiling Just Evaporation if the water was warmer, it would have evaporated faster. In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. the boiling temperature of a liquid is not the temperature at which it can enter the gaseous state. in an open container in an atmosphere where the vapor pressure. Water Not Boiling Just Evaporation.
From www.youtube.com
Difference between Evaporation and Boiling science learning academy Water Not Boiling Just Evaporation You will note from table that the vapor pressure of water at \(100^oc\) is \(1.01 \times 10^5 \, pa\), or 1.00 atm. if the water was warmer, it would have evaporated faster. in an open container in an atmosphere where the vapor pressure of water is below the equilibrium, there will continue. the boiling temperature of a. Water Not Boiling Just Evaporation.
From www.youtube.com
Evaporation VS Boiling YouTube Water Not Boiling Just Evaporation In contrast, boiling occurs only when the liquid reaches a certain temperature, which we call the boiling point. if air pressure is high on the surface of a body of water, then the water will not evaporate easily. why does water boil at \(100^oc\)? You will note from table that the vapor pressure of water at \(100^oc\) is. Water Not Boiling Just Evaporation.