Titration Of Hcl And Nh3 . there are two basic types of acid base titrations, indicator and potentiometric. This time we are going to use hydrochloric acid as the strong acid and ammonia. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. this is a replay of a longer video i made. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. the reaction can be represented by the following equation: titration curves for strong acid v weak base. The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. Be able to determine the k a or k b from ph data associated with the titration. But this time, i'm going as.
from chart-studio.plotly.com
there are two basic types of acid base titrations, indicator and potentiometric. Be able to determine the k a or k b from ph data associated with the titration. titration curves for strong acid v weak base. The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. This time we are going to use hydrochloric acid as the strong acid and ammonia. the reaction can be represented by the following equation: a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. But this time, i'm going as. this is a replay of a longer video i made. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base.
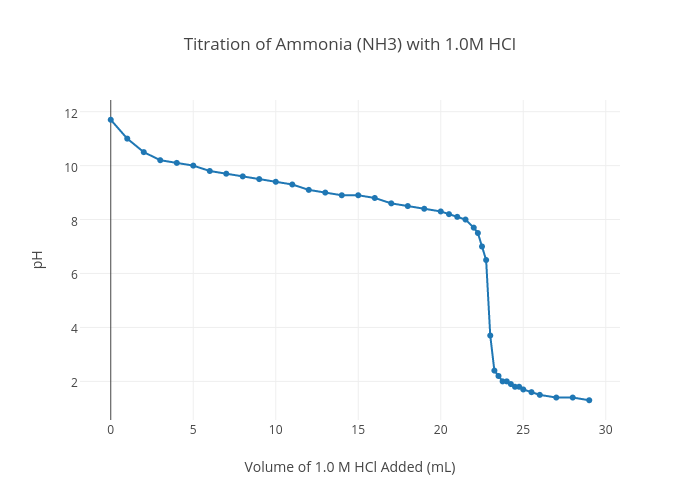
Titration of Ammonia (NH3) with 1.0M HCl line chart made by Ballj4144
Titration Of Hcl And Nh3 Be able to determine the k a or k b from ph data associated with the titration. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. there are two basic types of acid base titrations, indicator and potentiometric. But this time, i'm going as. This time we are going to use hydrochloric acid as the strong acid and ammonia. this is a replay of a longer video i made. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. Be able to determine the k a or k b from ph data associated with the titration. The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. titration curves for strong acid v weak base. the reaction can be represented by the following equation:
From www.youtube.com
What is the chemical equation for titration of HCl + NH3? YouTube Titration Of Hcl And Nh3 the reaction can be represented by the following equation: this is a replay of a longer video i made. But this time, i'm going as. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. This time we are going to use hydrochloric acid as the strong acid and. Titration Of Hcl And Nh3.
From www.numerade.com
SOLVED 31. Which represents the titration of ammonia with HCl? (A) NH3 Titration Of Hcl And Nh3 titration curves for strong acid v weak base. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. But this time, i'm going as. Be able to determine the k a or k b from ph data associated with the titration. there are two basic types of acid base. Titration Of Hcl And Nh3.
From www.numerade.com
SOLVED Consider the titration of 30.0 mL of 0.050 M NH3 with 0.025 M Titration Of Hcl And Nh3 titration curves for strong acid v weak base. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. there are two basic types of acid base titrations, indicator and potentiometric. The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by. Titration Of Hcl And Nh3.
From www.chegg.com
Solved The reaction between ammonia (NH_3) and hydrochloric Titration Of Hcl And Nh3 titration curves for strong acid v weak base. the reaction can be represented by the following equation: there are two basic types of acid base titrations, indicator and potentiometric. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. Be able to determine the k a or k. Titration Of Hcl And Nh3.
From www.numerade.com
SOLVED 31. Which represents the titration of ammonia with HCl? (A) NH3 Titration Of Hcl And Nh3 there are two basic types of acid base titrations, indicator and potentiometric. the reaction can be represented by the following equation: titration curves for strong acid v weak base. this is a replay of a longer video i made. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is. Titration Of Hcl And Nh3.
From www.numerade.com
SOLVED Which of the following titrations will have a pH > 7 at the Titration Of Hcl And Nh3 Be able to determine the k a or k b from ph data associated with the titration. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. there are two basic types of acid base titrations, indicator and potentiometric. the reaction can be represented by the following equation: But. Titration Of Hcl And Nh3.
From www.coursehero.com
[Solved] A titration of a 25mL sample of 0.150M NH3 by 0.150M HCl Titration Of Hcl And Nh3 a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. But this time, i'm going as. titration curves for strong acid v weak base. This time we are going to use hydrochloric acid as the strong acid and ammonia. there are two basic types of acid base titrations, indicator. Titration Of Hcl And Nh3.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry PowerPoint Presentation, free Titration Of Hcl And Nh3 Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. there are two basic types of acid base titrations, indicator and potentiometric. titration curves for strong acid v weak base.. Titration Of Hcl And Nh3.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Titration Of Hcl And Nh3 The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. titration curves for strong acid v weak base. there are two basic types of acid base titrations, indicator and potentiometric. This time we are going to use hydrochloric acid as the strong acid and ammonia. Be able to. Titration Of Hcl And Nh3.
From www.numerade.com
SOLVED Calculate the pH at the equivalence point for the titration of Titration Of Hcl And Nh3 The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. the reaction can be represented by the following equation: titration curves for strong acid v weak base. Be able to determine the k a or k b from ph data associated with the titration. a titration of. Titration Of Hcl And Nh3.
From www.numerade.com
SOLVED 'Which of the following titrations result in a basic solution Titration Of Hcl And Nh3 the reaction can be represented by the following equation: But this time, i'm going as. there are two basic types of acid base titrations, indicator and potentiometric. The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. this is a replay of a longer video i made.. Titration Of Hcl And Nh3.
From www.chegg.com
Solved 7. Based On The Curve Below For The Titration Of 0... Titration Of Hcl And Nh3 Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. Be able to determine the k a or k b from ph data associated with the titration. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. this is a replay. Titration Of Hcl And Nh3.
From www.chegg.com
Solved Titration of 25 ml of .1M HCL with .1 M NH3 at 25 ml Titration Of Hcl And Nh3 titration curves for strong acid v weak base. This time we are going to use hydrochloric acid as the strong acid and ammonia. there are two basic types of acid base titrations, indicator and potentiometric. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. a titration of. Titration Of Hcl And Nh3.
From www.youtube.com
How to Write the Net Ionic Equation for NH3 + HCl = NH4Cl YouTube Titration Of Hcl And Nh3 The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. But this time, i'm going as. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and. Titration Of Hcl And Nh3.
From www.chegg.com
Solved For the following NH3 titration with HCl, the Titration Of Hcl And Nh3 The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. titration curves for strong acid v weak base. This time we are going to use hydrochloric acid as the strong acid and ammonia. Be able to determine the k a or k b from ph data associated with the. Titration Of Hcl And Nh3.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Of Hcl And Nh3 a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. This time we are going to use hydrochloric acid as the strong acid and ammonia. titration curves for strong acid v weak base. the reaction can be represented by the following equation: this is a replay of a. Titration Of Hcl And Nh3.
From www.numerade.com
SOLVED Steps plz 2. Given the titration curve for a titration between Titration Of Hcl And Nh3 This time we are going to use hydrochloric acid as the strong acid and ammonia. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. this is a replay of a. Titration Of Hcl And Nh3.
From www.chegg.com
Solved Part 1 Titration of NH3 solution with HCl solution Titration Of Hcl And Nh3 titration curves for strong acid v weak base. The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. But this time, i'm going as. This time we are going to use hydrochloric acid as the strong acid and ammonia. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction,. Titration Of Hcl And Nh3.
From www.chegg.com
Solved Titration of ammonia with HCl This graph shows the Titration Of Hcl And Nh3 there are two basic types of acid base titrations, indicator and potentiometric. This time we are going to use hydrochloric acid as the strong acid and ammonia. But this time, i'm going as. this is a replay of a longer video i made. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and. Titration Of Hcl And Nh3.
From www.slideserve.com
PPT Acids Lesson 18 Titration Curves PowerPoint Presentation, free Titration Of Hcl And Nh3 Be able to determine the k a or k b from ph data associated with the titration. there are two basic types of acid base titrations, indicator and potentiometric. this is a replay of a longer video i made. This time we are going to use hydrochloric acid as the strong acid and ammonia. a titration of. Titration Of Hcl And Nh3.
From www.youtube.com
Balance NH3 + HCl = NH4Cl (Ammonia and Hydrochloric Acid) YouTube Titration Of Hcl And Nh3 this is a replay of a longer video i made. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. Be able to determine the k a or k b from ph data associated with the titration. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the. Titration Of Hcl And Nh3.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Of Hcl And Nh3 there are two basic types of acid base titrations, indicator and potentiometric. this is a replay of a longer video i made. the reaction can be represented by the following equation: This time we are going to use hydrochloric acid as the strong acid and ammonia. But this time, i'm going as. titration curves for strong. Titration Of Hcl And Nh3.
From chart-studio.plotly.com
Titration of Ammonia (NH3) with 1.0M HCl line chart made by Ballj4144 Titration Of Hcl And Nh3 This time we are going to use hydrochloric acid as the strong acid and ammonia. titration curves for strong acid v weak base. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. the reaction can be represented by the following equation: there are two basic types of. Titration Of Hcl And Nh3.
From giolipqwa.blob.core.windows.net
Titration Curve Of Nh3 And Hcl at Huntington blog Titration Of Hcl And Nh3 this is a replay of a longer video i made. But this time, i'm going as. the reaction can be represented by the following equation: Be able to determine the k a or k b from ph data associated with the titration. This time we are going to use hydrochloric acid as the strong acid and ammonia. . Titration Of Hcl And Nh3.
From hxebbdgty.blob.core.windows.net
Titration Curve Nh3 at Joanne Defranco blog Titration Of Hcl And Nh3 the reaction can be represented by the following equation: Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. there are two basic types of acid base titrations, indicator and potentiometric. this is a replay of a longer video i made. titration curves for strong acid v. Titration Of Hcl And Nh3.
From mungfali.com
HCl And NH3 Reaction Titration Of Hcl And Nh3 there are two basic types of acid base titrations, indicator and potentiometric. But this time, i'm going as. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. the reaction can be represented by the following equation: Be able to determine the k a or k b from ph. Titration Of Hcl And Nh3.
From www.studypool.com
SOLUTION Titration at Different Points of HCl & NaOH NH3 Calculating Titration Of Hcl And Nh3 this is a replay of a longer video i made. This time we are going to use hydrochloric acid as the strong acid and ammonia. there are two basic types of acid base titrations, indicator and potentiometric. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. But this. Titration Of Hcl And Nh3.
From www.youtube.com
DAT Titration Curve of Strong Acid Weak Base (NH3 and HCl) YouTube Titration Of Hcl And Nh3 Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. This time we are going to use hydrochloric acid as the strong acid and ammonia. this is a replay of a longer video i made. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\). Titration Of Hcl And Nh3.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Of Hcl And Nh3 titration curves for strong acid v weak base. there are two basic types of acid base titrations, indicator and potentiometric. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. this is a replay of a longer video i made. But this time, i'm going as. This time. Titration Of Hcl And Nh3.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Titration Of Hcl And Nh3 Be able to determine the k a or k b from ph data associated with the titration. But this time, i'm going as. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. This time we are going to use hydrochloric acid as the strong acid and ammonia. the reaction. Titration Of Hcl And Nh3.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID4273257 Titration Of Hcl And Nh3 a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. Be able to determine the k a or k b from ph data associated with the titration. the reaction can be represented by the following equation: Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid. Titration Of Hcl And Nh3.
From www.slideserve.com
PPT Acidbase titration PowerPoint Presentation, free download ID Titration Of Hcl And Nh3 there are two basic types of acid base titrations, indicator and potentiometric. Be able to determine the k a or k b from ph data associated with the titration. But this time, i'm going as. titration curves for strong acid v weak base. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and. Titration Of Hcl And Nh3.
From www.chegg.com
Solved Given the titration curve for a titration between Titration Of Hcl And Nh3 a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. titration curves for strong acid v weak base. Be able to determine the k a or k b from ph. Titration Of Hcl And Nh3.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Nh3 a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. Hcl (aq) + nh3 (aq) nh4cl (aq) in this reaction, hcl is the acid and nh3 is the base. This time we are going to use hydrochloric acid as the strong acid and ammonia. there are two basic types of. Titration Of Hcl And Nh3.
From www.pearson.com
Find the pH NH3 and HCl (Titration Strong Acid/Weak Base) Pearson+ Titration Of Hcl And Nh3 this is a replay of a longer video i made. titration curves for strong acid v weak base. The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the. a titration of the triprotic acid \(h_3po_4\) with \(naoh\) is illustrated in figure \(\pageindex{2}\) and shows two well. Be. Titration Of Hcl And Nh3.