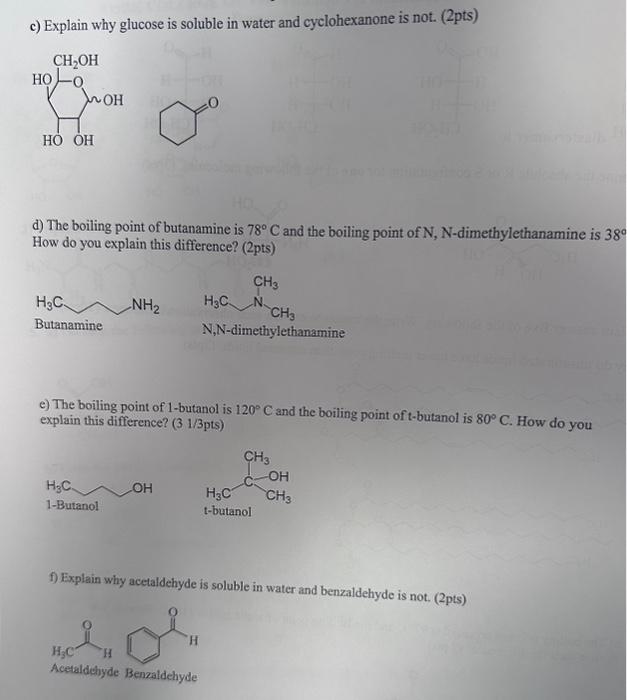
Why Do Unsaturated Fatty Acids Have A Lower Melting Point . Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. They may be saturated or unsaturated. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. Fatty acids are carboxylic acids that are the structural components of many lipids. Most fatty acids are unbranched and contain an even number of. Plants use unsaturated fatty acids to store energy. To illustrate this difference, the figure below shows the. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these.
from www.chegg.com
As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Most fatty acids are unbranched and contain an even number of. To illustrate this difference, the figure below shows the. Plants use unsaturated fatty acids to store energy. Fatty acids are carboxylic acids that are the structural components of many lipids. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. They may be saturated or unsaturated. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points.
Solved a) Why do unsaturated lipids have lower melting point
Why Do Unsaturated Fatty Acids Have A Lower Melting Point Fatty acids are carboxylic acids that are the structural components of many lipids. Plants use unsaturated fatty acids to store energy. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. To illustrate this difference, the figure below shows the. Most fatty acids are unbranched and contain an even number of. They may be saturated or unsaturated. Fatty acids are carboxylic acids that are the structural components of many lipids. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these.
From www.numerade.com
SOLVEDExplain why the melting points of unsaturated fatty acids are lower than those of Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Fatty acids are carboxylic acids that are the structural components of many lipids. Most fatty acids are unbranched and contain. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.chegg.com
Solved Unsaturated fatty acids have lower melting points Why Do Unsaturated Fatty Acids Have A Lower Melting Point Most fatty acids are unbranched and contain an even number of. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Fatty acids are carboxylic acids that are the structural components of many lipids. Since a double bond is stronger than a single bond, and the length of the c=c double bond is. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From slideplayer.com
Chapter 2 Lipids Lipids. ppt download Why Do Unsaturated Fatty Acids Have A Lower Melting Point Most fatty acids are unbranched and contain an even number of. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. To illustrate this difference, the figure below shows the.. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT Chapter 18 Lipids PowerPoint Presentation, free download ID793753 Why Do Unsaturated Fatty Acids Have A Lower Melting Point They may be saturated or unsaturated. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. To illustrate this difference, the figure below shows the. Most fatty. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.youtube.com
Scientific reason of how unsaturated fats have low melting point Part B Lecture1 By Dr Hadi Why Do Unsaturated Fatty Acids Have A Lower Melting Point Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Plants use unsaturated fatty acids to store energy. To illustrate this difference, the figure below shows the. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. Fatty acids are carboxylic acids that. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From slideplayer.com
Examples of Lipids. ppt download Why Do Unsaturated Fatty Acids Have A Lower Melting Point Fatty acids are carboxylic acids that are the structural components of many lipids. To illustrate this difference, the figure below shows the. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From egpat.com
Why stearic acid has more melting point than oleic acid? Why Do Unsaturated Fatty Acids Have A Lower Melting Point Fatty acids are carboxylic acids that are the structural components of many lipids. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. As a result, the attractive forces between. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT Face the Fats The Biochemistry of Lipids by Nancy A. Rice Western Kentucky University Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and.. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT Chapter 10.1 Storage Lipids PowerPoint Presentation, free download ID7063280 Why Do Unsaturated Fatty Acids Have A Lower Melting Point They may be saturated or unsaturated. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. Plants use unsaturated fatty acids to store energy. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. Unsaturated fatty acids. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.tuscany-diet.net
Fatty acids Tuscany Diet Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. To illustrate this difference, the figure below shows the. Plants use unsaturated fatty acids to store energy. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Most fatty acids are unbranched and. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT Lipids PowerPoint Presentation, free download ID4488229 Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. Most fatty acids are unbranched and contain an even number of. Fatty acids are carboxylic acids that are the structural components of many lipids. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From jesus-well-salazar.blogspot.com
Double Bonds in Fatty Acids Melting Point Why Do Unsaturated Fatty Acids Have A Lower Melting Point They may be saturated or unsaturated. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From quizlet.com
Diagram of Melting Points of Fatty Acids Diagram Quizlet Why Do Unsaturated Fatty Acids Have A Lower Melting Point Plants use unsaturated fatty acids to store energy. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. To illustrate this difference, the figure below shows the. Fatty acids are carboxylic acids that are the structural components of many lipids. Since a double bond is stronger than. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT Organic Chemistry 6 th Edition Paula Yurkanis Bruice PowerPoint Presentation ID3366327 Why Do Unsaturated Fatty Acids Have A Lower Melting Point Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. Fatty acids are carboxylic acids that are the structural components of many lipids. They may be saturated or unsaturated. Plants. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From laptrinhx.com
What is Unsaturated Fat? A Comprehensive Guide LaptrinhX / News Why Do Unsaturated Fatty Acids Have A Lower Melting Point Most fatty acids are unbranched and contain an even number of. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. Fatty acids are carboxylic acids that are the structural. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From slideplayer.com
Examples of Lipids. ppt download Why Do Unsaturated Fatty Acids Have A Lower Melting Point Most fatty acids are unbranched and contain an even number of. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. They may be saturated or unsaturated. Fatty acids are carboxylic acids that are the structural components of many lipids. As a result, the intermolecular attractions of. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.numerade.com
SOLVEDWhich of these fatty acids has the lower melting point? Explain why. (a) linoleic acid (b Why Do Unsaturated Fatty Acids Have A Lower Melting Point Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT LIPIDS PowerPoint Presentation, free download ID9343491 Why Do Unsaturated Fatty Acids Have A Lower Melting Point Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond is a liquid and. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From ibiologia.com
Fatty Acid Structure Examples Types Physical & Chemical Properties Why Do Unsaturated Fatty Acids Have A Lower Melting Point Plants use unsaturated fatty acids to store energy. To illustrate this difference, the figure below shows the. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Since a double bond is stronger. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT Chapter 18 Lipids PowerPoint Presentation, free download ID793753 Why Do Unsaturated Fatty Acids Have A Lower Melting Point Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Plants use unsaturated fatty acids to store energy. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. Since a double bond is stronger than a single bond, and the length of the c=c double bond. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.researchgate.net
6 Fatty acid chains and lipid molecules. (a) Saturated fatty acid... Download Scientific Diagram Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. Most fatty acids are unbranched and contain an even number of. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Fatty acids are carboxylic acids that are the structural components of many lipids. As a. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.researchgate.net
Melting points and enthalpies of individual fatty acids Download Table Why Do Unsaturated Fatty Acids Have A Lower Melting Point Plants use unsaturated fatty acids to store energy. They may be saturated or unsaturated. To illustrate this difference, the figure below shows the. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. Most fatty acids. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.numerade.com
The image shows lipid bilayer; with the polar heads represented by circles and the hydrophobic Why Do Unsaturated Fatty Acids Have A Lower Melting Point Fatty acids are carboxylic acids that are the structural components of many lipids. Most fatty acids are unbranched and contain an even number of. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond, why is it that the fat containing a double bond. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.researchgate.net
Representative chemical structure of saturated and unsaturated fatty... Download Scientific Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. To illustrate this difference, the figure below shows the. Unsaturated fatty acids have relatively low melting points, which explains why. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.numerade.com
SOLVED Describe why saturated fatty acids have higher melting points than do unsaturated fatty Why Do Unsaturated Fatty Acids Have A Lower Melting Point To illustrate this difference, the figure below shows the. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. Most fatty acids are unbranched and contain an even number of. They may be saturated or unsaturated. Since a double bond is stronger than a single bond, and the length of. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From courses.lumenlearning.com
Chapter 5. Biological Macromolecules Biology for Majors (openstax import) Why Do Unsaturated Fatty Acids Have A Lower Melting Point Plants use unsaturated fatty acids to store energy. Fatty acids are carboxylic acids that are the structural components of many lipids. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. As a result, the melting. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From savanna-has-underwood.blogspot.com
Double Bonds in Fatty Acids Melting Point SavannahasUnderwood Why Do Unsaturated Fatty Acids Have A Lower Melting Point Most fatty acids are unbranched and contain an even number of. Fatty acids are carboxylic acids that are the structural components of many lipids. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. To illustrate this difference, the figure below shows the. As a result, the attractive forces between. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From forums.studentdoctor.net
Boiling Point of fatty acids Student Doctor Network Why Do Unsaturated Fatty Acids Have A Lower Melting Point They may be saturated or unsaturated. Most fatty acids are unbranched and contain an even number of. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond,. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.chegg.com
Solved Part AWhy do unsaturated fats have lower melting Why Do Unsaturated Fatty Acids Have A Lower Melting Point Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. They may be saturated or unsaturated. Fatty acids are carboxylic acids that are the structural components of many lipids. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. To illustrate this difference, the figure below. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.researchgate.net
Effect of unsaturation and branched chains on lipid fluidity. The top... Download Scientific Why Do Unsaturated Fatty Acids Have A Lower Melting Point They may be saturated or unsaturated. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. To illustrate this difference, the figure below shows the. Plants use unsaturated fatty acids to store energy. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these.. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.chegg.com
Solved Unsaturated fatty acids have lower melting points Why Do Unsaturated Fatty Acids Have A Lower Melting Point Most fatty acids are unbranched and contain an even number of. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. Since a double bond is stronger than a single bond, and the length of the c=c double bond is shorter than that of the single bond,. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.visionlearning.com
Lipids Biology Visionlearning Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids.. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.chegg.com
Solved a) Why do unsaturated lipids have lower melting point Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids.. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT Lipids PowerPoint Presentation, free download ID6534192 Why Do Unsaturated Fatty Acids Have A Lower Melting Point As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. As a result, the attractive forces between unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. To illustrate this difference, the figure below shows the. Most fatty acids are unbranched and contain an. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.
From www.slideserve.com
PPT Lipids PowerPoint Presentation, free download ID6534192 Why Do Unsaturated Fatty Acids Have A Lower Melting Point They may be saturated or unsaturated. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these. Most fatty acids are unbranched and contain an even number of. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. To illustrate this difference, the figure. Why Do Unsaturated Fatty Acids Have A Lower Melting Point.