Atomic Radius Rules . Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. What is the trend for atomic radius? Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron.
from periodictableguide.com
The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. What is the trend for atomic radius? Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots.
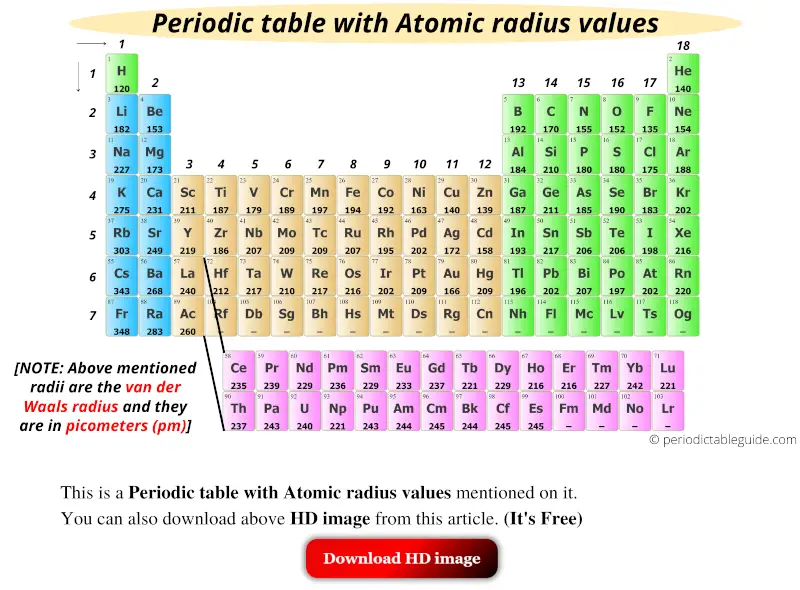
Get the Periodic table with Atomic radius values (Img+Chart)
Atomic Radius Rules Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. What is the trend for atomic radius? Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together.
From sciencenotes.org
Atomic Radius and Ionic Radius Atomic Radius Rules What is the trend for atomic radius? Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. The atomic radius is measured based on the distance between the nuclei of two atoms that. Atomic Radius Rules.
From www.yaclass.in
Atomic Radius and Ionic Radii — lesson. Science State Board, Class 10. Atomic Radius Rules Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means. Atomic Radius Rules.
From utedzz.blogspot.com
Atomic Radius Periodic Table Electronegativity Periodic Table Timeline Atomic Radius Rules 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Learn the two rules you need to know and how to use the atomic radius trend to predict. Atomic Radius Rules.
From blog.prepscholar.com
Understanding Atomic Radius Trends The 2 Key Principles · PrepScholar Atomic Radius Rules What is the trend for atomic radius? Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded. Atomic Radius Rules.
From www.slideserve.com
PPT PERIODIC TRENDS 1. ATOMIC RADIUS 2. IONIC RADIUS PowerPoint Atomic Radius Rules Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. The atomic radius is measured based on the distance between the nuclei of. Atomic Radius Rules.
From sciencenotes.org
Atomic Radius and Ionic Radius Atomic Radius Rules The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Learn the two rules you. Atomic Radius Rules.
From www.slideserve.com
PPT Atomic Radius PowerPoint Presentation, free download ID2100315 Atomic Radius Rules Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The covalent atomic radius (r cov). Atomic Radius Rules.
From www.slideserve.com
PPT Atomic Radius PowerPoint Presentation, free download ID2100315 Atomic Radius Rules Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The atomic radius is measured based. Atomic Radius Rules.
From www.slideserve.com
PPT II. Periodic Table PowerPoint Presentation, free download ID Atomic Radius Rules The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms. Atomic Radius Rules.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) Atomic Radius Rules 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. What is the trend for atomic radius? The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the. Atomic Radius Rules.
From www.youtube.com
Atomic Radius Types of atomic radius S Chemistry Live YouTube Atomic Radius Rules The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. What is the trend for atomic radius? Atomic radius is. Atomic Radius Rules.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) Atomic Radius Rules What is the trend for atomic radius? Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. 119 rows the atomic radius of a chemical element is the distance from the center of. Atomic Radius Rules.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps Atomic Radius Rules The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Learn the two rules you. Atomic Radius Rules.
From www.slideserve.com
PPT Atomic Radius PowerPoint Presentation, free download ID2100315 Atomic Radius Rules Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. What is the trend for atomic radius? Learn the two rules you. Atomic Radius Rules.
From periodictrendsforhonorschemistry.weebly.com
Atomic Radius Trends of the Periodic Table Atomic Radius Rules 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms. Atomic Radius Rules.
From www.slideserve.com
PPT Chapter 6 Ionic Bonds and Some Main Group Chemistry PowerPoint Atomic Radius Rules Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. What is the trend for atomic radius? The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. Learn the two rules you need. Atomic Radius Rules.
From www.breakingatom.com
Atomic Radius of Elements Atomic Radius Rules Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. 119 rows the atomic radius of a chemical element. Atomic Radius Rules.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General Atomic Radius Rules Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. 119 rows the atomic radius of a chemical element. Atomic Radius Rules.
From study.com
What is Atomic Radius? Atomic Radius Examples & Periodic Trend Atomic Radius Rules Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. Atomic Radius Rules.
From www.slideserve.com
PPT Chemical Bonding Bonding Theory and Lewis Formulas PowerPoint Atomic Radius Rules Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Explore how atomic radius changes with atomic number in the periodic table of elements via. Atomic Radius Rules.
From ezchem.com
Atomic Radius Trend EZchem Atomic Radius Rules Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. What is the trend for atomic radius? The atomic radius is measured based. Atomic Radius Rules.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 Atomic Radius Rules The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. What is the trend for atomic radius? 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of. Atomic Radius Rules.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Atomic Radius Rules 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely. Atomic Radius Rules.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Atomic Radius Rules The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Learn the two rules you. Atomic Radius Rules.
From www.youtube.com
Periodic Classification Of Elements Part 6 Slater Rules Trends In Atomic Radius Rules Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. What is the trend for atomic radius? 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to. Atomic Radius Rules.
From www.sliderbase.com
Periodic Behavior Presentation Chemistry Atomic Radius Rules 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. Learn the two rules you. Atomic Radius Rules.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Atomic Radius Rules The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Learn the two rules you need to know and how to use. Atomic Radius Rules.
From 88guru.com
Atomic RadiusAn Overview 88Guru Atomic Radius Rules Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The covalent atomic radius (r cov). Atomic Radius Rules.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Atomic Radius Rules 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. Learn the two rules. Atomic Radius Rules.
From www.slideserve.com
PPT PERIODIC TRENDS 1. ATOMIC RADIUS 2. IONIC RADIUS PowerPoint Atomic Radius Rules The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The atomic radius is measured based on the distance between the nuclei of. Atomic Radius Rules.
From www.ck12.org
Atomic Radius Example 2 ( Video ) Chemistry CK12 Foundation Atomic Radius Rules The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The covalent atomic radius (r cov) is half the internuclear distance in. Atomic Radius Rules.
From www.youtube.com
Atomic Radius Basic Introduction Periodic Table Trends, Chemistry Atomic Radius Rules Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. What is the trend for atomic radius? 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. The atomic radius is measured based on the. Atomic Radius Rules.
From flexbooks.ck12.org
CK12Foundation Atomic Radius Rules Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. Learn the two rules you need to know and how to use the. Atomic Radius Rules.
From byjus.com
Atomic Radius Definition, Types, Periodic Trends of Atomic Radii Atomic Radius Rules What is the trend for atomic radius? The atomic radius is measured based on the distance between the nuclei of two atoms that are barely touching each other, which means the electron shells of the two atoms are. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other,. Atomic Radius Rules.
From periodicitychem.weebly.com
Atomic Radius Periodicity Project Atomic Radius Rules Learn the two rules you need to know and how to use the atomic radius trend to predict atom size. Explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. Atomic Radius Rules.